Abstract
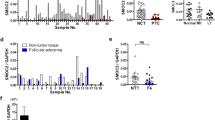
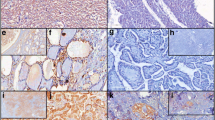
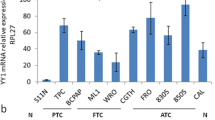
Smad proteins have been shown to mediate the signal transduction pathway downstream of the transforming growth factor β (TGFβ). TGFβ induces the phosphorylation of Smad2 and Smad3 which associate with Smad4 and translocate to the nucleus where they regulate gene transcription; besides these stimulatory Smads, the inhibitory Smads, Smad6 and Smad7, oppose signaling by blocking receptors and interrupting the phosphorylation of Smads2/3. The loss of TGFβ-sensitivity, caused by inactivation of components of TGFβ signaling, as Smad4, underlies a wide variety of human disorders, including cancer. In addition, the overexpression of the inhibitory Smad7, which prevents the phosphorylation of Smad2/3 and consequently inhibits TGFβ signaling pathways, was observed in some diseases. In the present study we investigated the expression of Smad4 and Smad7 in thyroid cell lines (NPA papillary carcinoma, WRO follicular carcinoma and ARO anaplastic carcinoma) by RT-PCR and immunocytochemistry. Our results show that Smad4 was expressed in all thyroid cell lines and controls analyzed, differently from other classes of tumors where Smad4 expression was deleted. On the other hand, Smad7 was overexpressed in ARO anaplastic cell line, the most malignant follicular thyroid carcinoma. Our data suggest that the abrogation of the TGFβ response by Smad7 overexpression may be a mechanism for the tumor aggressiveness observed in undifferentiated thyroid tumors.
Similar content being viewed by others
References
Heldin C.H., Miyazono K., ten Dijke P. TGF-β signaling from cell membrane to nucleus through Smad proteins. Nature 1997, 390: 465–471.
Massagué J., Blain S.W., Lo R.S. TGF β signaling in growth control, cancer, and heritable disorders. Cell 2000, 103: 295–309.
Blobe C.G, Schieldmann W.P, Lodish H.F. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000, 342: 1350–1358.
Wrana J.L. Regulation of Smad activity. Cell 2000, 100: 189–192.
Attisano L., Lee-Hoeflich S.T. The Smads. Genome Biology 2001, 2: 3010.1–3010.8.
Nakao A., Afrakhte M., Morén A., et al. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 1997, 389: 631–635.
Hölting T., Zielke A., Siperstein A.E., Clark O.H., Duh Q-Y. Transforming growth factor-β1 is a negative regulator for differentiated thyroid cancer: studies of growth, migration, invasion, and adhesion of cultured follicular and papillary thyroid cancer cell lines. J. Clin. Endocrinol. Metab. 1995, 79: 806–813.
Markowitz S., Wang J., Myeroff L., et al. Inactivation of the type II TGFβ receptor in colon cancer cells with microsatellite instability. Science 1995, 268: 1336–1338.
Hahn S.A., Schutte M., Hoque A.T.M.S., et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996, 271: 350–353.
Riggins G.J., Kinzler K.W., Vogelstein B., Thiagalingam S. Frequency of Smad mutations in human cancers. Cancer Res. 1997, 57: 2578–2580.
Kimura E.T., Kopp P., Zbaeren J., et al. Expression of transforming growth factor β1, β2, and β3 in multinodular goiters and differentiated thyroid carcinomas: a comparative study. Thyroid 1999, 9: 119–125.
Ohta K., Pang X.P., Berg L., Hershman J.M. Antitumor actions of cytokines on new human papillary thyroid carcinoma cell lines. J. Clin. Endocrinol. Metab. 1996, 81: 2607–2612.
Asmis L.M., Kaempf J., Von Gruenigen C., Kimura E.T., Wagner H.E., Studer H. Acquired and naturally occurring resistance of thyroid follicular cells to the growth inhibitory action of transforming growth factor-beta 1 (TGF-β1). J. Endocrinol. 1996, 149: 485–496.
Estour A.J., Van Herle G.J.F., Juillard T.L., et al. Characterization of a human follicular thyroid carcinoma cell line (UCLA RO 82 W-1). Virchows Arch. [Cell. Pathol.] 1989, 57: 167–174.
Pang X.J., Hershmann J.M., Chung M., Pekary A.E. Characterization of tumor necrosis factor-a receptors in human and rat thyroid cells and regulation of the receptors by thyrotropin. Endocrinology 1989, 125: 1783–1788.
Biscolla R.P.M., Cerutti J.M., Maciel R.M.B. Detection of recurrent thyroid cancer by sensitive nested RT-PCR of thyroglobulin and sodium/iodine symporter messenger RNA transcripts in peripheral blood. J. Clin. Endocrinol. Metabol. 2000, 85: 3623–3627.
Park K., Kim S.J., Bang Y., Park J.K., Roberts A.B., Sporn M.P. Genetic changes in the transforming growth factor β (TGF-β) type II receptor gene in human gastric cancer cells: correlation with sensitivity to growth inhibition by TGF-β. Proc. Natl. Acad. Sci. USA 1994, 91: 8772–8776.
Cowin A.J., Davis J.R.E., Bidey S.P. Transforming growth factor-β1 production in porcine thyroid follicular cells: regulation by intrathyroidal organic iodine. J. Mol. Endocrinol. 1992, 9: 197–205.
Grubeck-Loebenstein B., Buchan G., Sadeghi R. et al. Transforming growth factor beta regulates thyroid growth. Role in the pathogenesis of nontoxic goiter. J. Clin. Invest. 1989, 83: 764–770.
Lazzareschi D., Ranieri A., Mincione G., Taccogna S., Nardi F., Colleta G. Human malignant thyroid tumors displayed reduced levels of transforming growth factor β receptor type II messenger RNA and protein. Cancer Res. 1997, 57: 2071–2076.
West J., Munoz-Antonia T., Johnson J.G., Klotch D., Muro-Cacho C.A. Transforming growth factor-β type II receptors and Smad proteins in follicular thyroid tumors. Laryngoscope 2000, 110: 1323–1327.
Heldin N.E., Bergström D., Hermansson A., et al. Lack of responsiveness to TGF-β1 in a thyroid carcinoma cell line with functional type I and type II TGF-β receptors and Smad proteins, suggests a novel mechanism for TGF-β insensitivity in carcinoma cells. Mol. Cell. Endocrinol. 1999, 153: 79–90.
Chen C-H., Kang Y., Massagué J. Defective repression of c-myc in breast cancer cells: A loss at the core of the transforming growth factor β growth arrest program. Proc. Natl. Acad. Sci. USA 2001, 98: 992–999.
Pientepol J.A., Münger K., Howley P.M., Stein R.W., Moses H.L. Factor-binding element in the human c-myc promoter involved in transcriptional regulation by transforming growth factor β1 and by the retinoblastoma gene product. Proc. Natl. Acad. Sci. USA 1991, 88: 10227–10231.
Usa T., Tsukazaki T., Namba H., et al. Correlation between suppression of c-myc and antiproliferative effect of transforming growth factor-β1 in thyroid carcinoma cell growth. Endocrinology 1994, 135: 1378–1384.
Cerutti J.M., Trapasso F., Battaglia C., et al. Block of cmyc expression by antisense oligonucleotides inhibits proliferation of human thyroid carcinoma cell lines. Clin. Cancer Res. 1996, 2: 119–126.
Visconti R., Cerutti J.M., Battista S., et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFκβ p65 protein expression. Oncogene 1997, 15: 1987–1994.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cerutti, J.M., Ebina, K.N., Matsuo, S.E. et al. Expression of Smad4 and Smad7 in human thyroid follicular carcinoma cell lines. J Endocrinol Invest 26, 516–521 (2003). https://doi.org/10.1007/BF03345213
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345213