Abstract
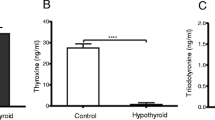
Thyroid hormone is known to play a pivotal role in the regulation of prepuberal rat testes development and function with specific influence on the differentiation of Sertoli cells, the only cell type that expresses thyroid hormone receptors in testes. To explore in vivo effects of thyroid hormone on testes development and the regulation of testicular gene expression, the hyperand hypothyroid rat models were established by T3 injection to pups (ip 100 μg/kg bw) and by oral administration of 6-N-propyl-2-thiouracil (PTU) to the lactating mother from days 1 to 21 post-delivery. Half of the rats from each group were sacrificed at 21 days of age, and the other half were allowed to recover with discontinued treatments from day 22 to day 50. At 21 days of age, a significantly elevated serum T3 level was observed in hyperthyroid rats (179.5 ng/dl) vs controls (97.5 ng/dl), and in hypothyroid rats a significantly lower level of T3 was detected (26.1 ng/dl). However, serum T4 concentration was significantly lower in both hyper- (0.105 μg/dl) and hypothyroid (0.058 μg/dl) rats compared to the controls (2.48 μg/dl). In recovered rats in which the serum T3 and T4 were restored to normal, the serum T levels remained remarkably lower in both hyper- and hypothyroid rats. The significantly decreased body and testes weights observed in both hyper- and hypothyroid rats at 21 days of age were not restored by the time they were 50 days old. Histological analyses of testes of 21-day-old hypothyroid rats revealed smaller-sized seminiferous tubules, incomplete lumen formation and delayed germ cell differentiation and in hyperthyroid rats an increased number of early stage spermatocytes was found. Testicular mRNA levels of follicle-stimulating hormone receptor (FSH-R), luteinizing hormone receptor (LH-R) and androgen binding protein (ABP) were studied by Northern blot hybridization. At 21 days of age data showed that FSH-R mRNA levels were significantly higher in both hyper- and hypothyroid rat testes compared to controls, but no differences were detected in recovered 50-day-old rats. Significantly decreased ABP mRNA levels were detected only in hypothyroid rat testes compared to those in both the hyperthyroid and control groups at 21 days of age, but no significant change was observed in recovered 50-day-old rats. To further evaluate the effect of thyroid hormone on the Leydig cell function, the 2.3/2.6 kb specific LH-R hybridization bands were detected with rat LH-R cRNA probe. Significant suppression of LH-R mRNA levels was only observed in the hypothyroid rat testes at 50 days of age. The testicular thyroid hormone receptors (TRs) and the regulation of TR by thyroid hormone were investigated using semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) assays. Both TRα and TRβ mRNAs were identified in the testes from 21- and/or 50- day-old rats. TRα mRNA levels were significantly increased in hypothyroid rat testes and were suppressed in hyperthyroid rats at 21 days of age and no changes of TRα mRNA were found in recovered animals. Our in vivo data strongly suggest that the thyroid hormone directly affects the development of prepuberal testes and the regulation of FSH-R and ABP gene expression in Sertoli cells, as well as the LH-R mRNA levels in Leydig cells, which may lead to further modulating the effect of gonadotropins on testes function.
Similar content being viewed by others
References
Goldman S., Dirnfeld M., Abramovici H., Kraiem Z. Triiodothyronine (T3) modulates hCG-regulated progesterone secretion, cAMP accumulation and DNA content in cultured human luteinized granulosa cells. Mol. Cell. Endocrinol. 1993, 96: 125–131.
Van Voorhis B.J., Neff T.W., Syrop C.H., Chapler F.K. Primary hypothyroidism associated with multicystic ovaries and ovarian torsion in an adult. Obstet. Gynecol. 1994, 83: 885–887.
Jannini E.A., Carosa E., Rucci N., Screponi E., D’Armiento M. Ontogeny and regulation of variant thyroid hormone receptor isoforms in developing rat testis. J. Endocrinol. Invest. 1999, 22: 843–848.
Jannini E.A., Dolci S., Ulisse S., Nikodem V.M. Developmental regulation of the thyroid hormone receptor α 1 mRNA expression in the rat testes. Mol. Endocrinol. 1994, 8: 89–96.
Arambepola N.K., Bunick D., Cooke P.S. Thyroid hormone and follicle-stimulating hormone regulate mullerian inhibiting substance messenger ribonucleic acid expression in cultured neonatal rat Sertoli cells. Endocrinology 1998, 139: 4489–4495.
Ando S., Sirianni R., Forastieri P., et al. Aromatase expression in prepuberal Sertoli cells: effect of thyroid hormone. Mol. Cell. Endocrinol. 2001, 178: 11–21.
Heckert L.L., Griswold M.D. Expression of follicle-stimulating hormone receptor mRNA in rat testes and Sertoli cells. Mol. Endocrinol. 1991, 5: 670–677.
Tena-Sempere M., Zhang F.P., Huhtaniemi I. Persistent expression of a truncated form of the luteinizing hormone receptor messenger ribonucleic acid in the rat testes after selective Leydig cell destruction by ethylene dimethane sulfonate. Endocrinology 1994, 135: 1018–1024.
Anthony C.T., Danzo B.J., Orgebin-Crist M.C. Investigations on the relationship between sperm fertilizing ability and androgen-binding protein in the hypophysectomized, pregnenoloneenolone-injected rat. Endocrinology 1984, 114: 1419–1425.
Holland M.K., Rogers B.J., Orgebin-Crist M.C., Danzo B.J. Effects of photoperiod on androgen-binding protein and sperm fertilizing ability in the hamster. J. Reprod. Fertil. 1987, 81: 99–112.
Majdic G., Sno J., Horvat A., Mrkun J., Kosec M., Cestnik V. Higher thyroid hormone levels in neonatal life result in reduced testis volume in postpubertal bulls. Int. J. Androl. 1998, 21: 352–357.
Kirby J.D., Jetton A.E., Cooke P.S., et al. Developmental hormonal profiles accompanying the neonatal hypothyroidism-induced increase in adult testicular size and sperm production in the rat. Endocrinology 1992, 131: 559–565.
Tamasy V., Du j-Zm Vallerga A., Meisami E., Timiras P.S. Suckling ability and maternal prolactin levels in hypothyroid rats. Horm. Behav. 1984, 18: 457–464.
Trainer T.D. Histology of the normal testes. Am. J. surgical. Pathol. 1987, 11: 797–809.
Cooke P.S., Meisami E. Early hypothyroidism in rats causes increased adult testes and reproductive organ size but does not change testosterone levels. Endocrinology 1991, 129: 237–243.
Rao J.N., Chandrashekar V., Borg K.E., Bartke A. Effects of photoperiod on testicular inhibin-a and androgen binding protein mRNA expression during postnatal development in Siberian hamsters, Phodopus sungorus. Life Sci. 1995, 57: 1761–1770.
Juszczak M., Steger R.W., Debeljuk L., et al. The effects of short-photoperiod, pinealectomy and melatonin treatment on oxytocin synthesis and release in the male Syrian hamster. Endocrine 1996, 4: 223–231.
Chomaczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162: 156–159.
Lad R.P., Smith M.A., Hilt D.C. Molecular cloning and regional distribution of rat brain cyclophilin. Mol. Brain Res. 1991, 9: 239–244.
El-Husseini A.E.D., Paterson J.A., Shiu R.P.C. Basic fibroblast growth factor (BFGF) and two of its receptors, FGFR1 and FGFR2: gene expression in the rat brain during postnatal development as determined by quantitative RTPCR. Mol. Cell Endocrinol. 1994, 104: 191–200.
Sheflin L.G., Fucile N.W., Ozawa S., Spaulding S.W. Thyroxine increases the levels of epidermal growth factor messenger ribonucleic acid (EGF mRNA) in the thyroid in vivo, as revealed by quantitative reverse transcription polymerase chain reaction with an internal control EGF mRNA. Endocrinology 1993, 132: 2319–2324.
Bruni J.F., Marshall S., Dibbet J.A., Meites J. Effects of hyper and hypothyroidism on serum LH and FSH levels in intact and gonadectomized male and female rats. Endocrinology 1975, 97: 558–563.
Ruiz M., Diego A.M., Reyes A., Alonso A., Morell M. Influence of thyroidectomy on serum and pituitary FSH in control and orchidectomized rats. Res. Exp. Med. (Berl.) 1989, 189: 85–90.
Hardy M.P., Kirby J.D., Hess R.A., Cooke P.S. Leydig cells increase their numbers but decline in steroidogenic function in the adult rat after neonatal hypothyroidism. Endocrinology 1993, 132: 2417–2420.
Baksi S.N. Effect of propylthiouracil-induced hypothyroidism on serum levels of luteinizing hormone and follicle-stimulating hormone in the rat. J. Endocrinol. 1973, 59: 655–656.
Aruldhas M.M., Valivullah H.M., Govindarajulu P. Specific effect of thyroid hormone testicular enzymes involved in carbohydrate metabolism. Biochem. Biophys. Acta 1982, 715: 121–125.
Panno M.L., Salerno M., Lanzino M., et al. Follow-up study on the effects of thyroid hormone administration on androgen metabolism of peripubertal rat Sertoli cells. Eur. J. Endocrinol. 1995, 132: 236–241.
Valle L.B.S., Oliveira-Filho R.M., Romaldini J.H., Lara P.F. Pituitary-testicular axis abnormalities in immature male hypothyroid rats. J. Steroid Biochem. 1985, 23: 253–257.
Van Haaster L.H., De Jong F.H., Docter R., De Rooij D.G. The effect of hypothyroidism on Sertoli cell proliferation and differentiation and hormone levels during testicular development in the rat. Endocrinology 1992, 131: 1574–1576.
Palmero S., Prati M., Bolla F., Fugassa E. Tri-iodothyronine directly affects rat Sertoli cell proliferation and differentiation. J. Endocrinol. 1995, 145: 355–362.
Maran R.R., Sivakumar R., Arunakaran J., et al. Durationdependent effect of transient neonatal hypothyroidism on Sertoli and germ cell number, and plasma and testicular interstitial fluid androgen binding protein concentration. Endocr. Res. 1999, 25: 323–340.
French F.S., Ritzen E.M. A high affinity androgen binding protein (ABP) in rat testes: evidence for secretion into efferent duct fluid and absorption by epididymis. Endocrinology 1973, 93: 88–95.
Fugassa E., Palmero S., Gallo G. Triiodothyronine decreases the production of androgen binding globulin by rat Sertoli cells. Biochem. Biophys. Res. Commun. 1987, 143: 241–247.
Hess R.A., Cooke P.S., Bunick D., Kirby J.D. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology 1993, 132: 2607–2613.
Palmero S., Maggiani S., Fugassa E. Nuclear triiodothyronine receptors in rat Sertoli cells. Mol. Cell Endocrinol. 1988, 58: 253–256.
Jannini E.A., Olivieri M., Francavilla S., Gulino A., Ziparo E., D’Armiento M. Ontogenesis of the nuclear 3,5,3’-triiodothyronine receptor in the rat testes. Endocrinology 1990, 126: 521–2526.
Evans R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240: 889–895.
Lazar M.A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr. Rev. 1993, 14: 184–193.
Palmero S., De Marco P., Fugassa E. Thyroid hormone receptor β mRNA in Sertoli cells isolated from prepuberal testes. J. Endocrinol. 1995, 14: 131–134.
Bunick D., Kirby J., Hess R.A., Cooke P.S. Developmental expression of testes messenger ribonucleic acids in the rat following prophylthiouracil-induced neonatal hypothyroidism. Biol. Reprod. 1994, 51: 706–713.
Jannini E.A., Ulisse S., D’Armiento M. Thyroid hormone and male gonadal function. Endocr. Rev. 1995, 16: 443–459.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, J.N., Liang, J.Y., Chakraborti, P. et al. Effect of thyroid hormone on the development and gene expression of hormone receptors in rat testes in vivo . J Endocrinol Invest 26, 435–443 (2003). https://doi.org/10.1007/BF03345199
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345199