Abstract
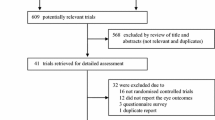
SS analogs are an attractive alternative in treating Graves’ ophthalmopathy (GO). Most of the previous studies were uncontrolled and enrolled few patients. The present study was conducted as a larger scale, prospective, randomized controlled study to determine the effectiveness of a slow-release formulation of lanreotide in GO. Sixty patients with active GO received an im injection every two weeks of either lanreotide 30 mg or placebo for 12 weeks. They were then followed and further treated in the traditional way if necessary. The Clinical Activity Score (CAS) was the primary efficacy criterion. Proptosis, diplopia, corneal erosion or ulcer, visual acuity, extraocular muscle movement and intraocular pressure were also evaluated. At the end of the 12 weeks, the mean CAS was not significantly decreased in the lanreotide group compared to the placebo group. The overall mean difference of proptosis between these two groups also did not reach significance at 12 weeks. Only diplopia at downward gaze had significant improvement for the lanreotide-treated group vs placebo group (p=0.03). No differences were observed between the two groups compared to other outcome measures. During the 24-month follow-up after the clinical trial, 14 patients received eye surgery in the placebo group compared with 10 patients in the lanreotide group (p=0.29). Six patients received methylprednisolone pulse therapy in the placebo group and two patients in the lanreotide group (p=0.25). In conclusion, lanreotide treatment had no significant effects on GO compared with placebo.
Similar content being viewed by others
References
Marcocci C, Bartalena L, Bogazzi F, Panicucci M, Pinchera A. Studies on the occurrence of ophthalmopathy in Graves’ disease. Acta Endocrinol (Copenh) 1989, 120: 473–8.
Bahn RS. Pathophysiology of Graves’ ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab 2003, 88: 1939–46.
Bahn RS, Heufelder AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med 1993, 329: 1468–75.
Diez JJ. Therapy of Graves’ ophthalmopathy: a novel application of somatostatin analogues. Expert Opin Pharmacother 2001, 2: 1361–5.
Heufelder AE, Spitzweg C. Immunology of Graves’ ophthalmopathy. Dev Ophthalmol 1999, 30: 24–38.
Kahaly G, Forster G, Hansen C. Glycosaminoglycans in thyroid eye disease. Thyroid 1998, 8: 429–32.
Krassas GE. Somatostatin analogues in the treatment of thyroid eye disease. Thyroid 1998, 8: 443–5.
Chang TC, Kao SC, Huang KM. Octreotide and Graves’ ophthalmopathy and pretibial myxoedema. BMJ 1992, 304: 158.
Ozata M, Bolu E, Sengul A, et al. Effects of octreotide treatment on Graves’ ophthalmopathy and circulating sICAM-1 levels. Thyroid 1996, 6: 283–8.
Krassas GE. Somatostatin analogs: a new tool for the management of Graves’ ophthalmopathy. J Endocrinol Invest 2004, 27: 281–7.
Kahaly G, Diaz M, Just M, Beyer J, Lieb W. Role of octreo-scan and correlation with MR imaging in Graves’ ophthalmopathy. Thyroid 1995, 5: 107–11.
Krassas GE, Dumas A, Pontikides N, Kaltsas T. Somatostatin receptor scintigraphy and octreotide treatment in patients with thyroid eye disease. Clin Endocrinol (Oxf) 1995, 42: 571–5.
Postema PT, Krenning EP, Wijngaarde R, et al. (111In-DTPA-D-Phe1)octreotide scintigraphy in thyroidal and orbital Graves’ disease: a parameter for disease activity? J Clin Endocrinol Metab 1994, 79: 1845–51.
Pasquali D, Notaro A, Bonavolontà G, Vassallo P, Bellastella A, Sinisi AA. Somatostatin receptor genes are expressed in lymphocytes from retroorbital tissues in Graves’ disease. J Clin Endocrinol Metab 2002, 87: 5125–9.
Pasquali D, Vassallo P, Esposito D, Bonavolontà G, Bellastella A, Sinisi AA. Somatostatin receptor gene expression and inhibitory effects of octreotide on primary cultures of orbital fibroblasts from Graves’ ophthalmopathy. J Mol Endocrinol 2000, 25: 63–71.
International ad hoc committee. Classification of eye changes of Graves’ disease. Thyroid 1992, 2: 235–6.
Werner SC. Modification of the classification of the eye changes of Graves’ disease: recommendations of the ad hoc committee of the American Thyroid Association. J Clin Endocrinol Metab 1977, 44: 203–4.
Hettmansperger TP, McKean J. Robust nonparametric statistical methods. London: Arnold. 1998.
Krassas GE, Kaltsas T, Dumas A, Pontikides N, Tolis G. Lanreotide in the treatment of patients with thyroid eye disease. Eur J Endocrinol 1997, 136: 416–22.
Krassas GE, Doumas A, Kaltsas T, Halkias A, Pontikides N. Somatostatin receptor scintigraphy before and after treatment with somatostatin analogues in patients with thyroid eye disease. Thyroid 1999, 9: 47–52.
Kahaly G, Diaz M, Hahn K, Beyer J, Bockisch A. Indium-111-pentreotide scintigraphy in Graves’ ophthalmopathy. J Nucl Med 1995, 36: 550–4.
Colao A, Pivonello R, Lastoria S, et al. Clinical implications of somatostatin-receptor scintigraphy in ophthalmic Graves’ disease. Eur J Endocrinol 2000, 143: S35–42.
Gerding MN, van der Zant FM, van Royen EA, et al. Octreotide-scintigraphy is a disease-activity parameter in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1999, 50: 373–9.
Uysal AR, Corapcioglu D, Tonyukuk VC, et al. Effect of octreotide treatment on Graves’ ophthalmopathy. Endocr J 1999, 46: 573–7.
Kung AW, Michon J, Tai KS, Chan FL. The effect of somatostatin versus corticosteroid in the treatment of Graves’ ophthalmopathy. Thyroid 1996, 6: 381–4.
Wemeau JL, Caron P, Beckers A, et al. Octreotide (long-acting release formulation) treatment in patients with Graves’ orbitopathy: clinical results of a four-month, randomized, placebo-controlled, double-blind study. J Clin Endocrinol Metab 2005, 90: 841–8.
Dickinson AJ, Vaidya B, Miller M, et al. Double-blind, placebo-controlled trial of octreotide long-acting repeatable (LAR) in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2004, 89: 5910–5.
Terwee CB, Prummel MF, Gerding MN, et al. Measuring disease activity to predict therapeutic outcome in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 2005, 62: 145–55.
Feldon S. Classification of Graves’ ophthalmopathy. Thyroid 1993, 3: 171.
Gerding M, Prummel M, Wiersinga W. Assessment of disease activity in Graves’ ophthalmopathy by orbital ultra-sonography and clinical parameters. Clin Endocrinol (Oxf) 2000, 52: 641–6.
Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol 1989, 73: 639–44.
Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1997, 47: 9–14.
Dickinson AJ, Perros P. Controversies in the clinical evaluation of active thyroid-associated orbitopathy: use of a detailed protocol with comparative photographs for objective assessment. Clin Endocrinol (Oxf) 2001, 55: 283–303.
Chang TC, Huang KM, Hsiao YL, et al. Relationships of orbital computed tomographic findings and activity scores to the prognosis of corticosteroid therapy in patients with Graves’ ophthalmopathy. Acta Ophthalmol Scand 1997, 75: 301–4.
Balazs C, Bokk A, Farid NR. Serum soluble CD8 concentration is an indicator of disease activity in patients with Graves’ disease. Thyroid 1994, 4: 27–30.
Prummel MF, Gerding MN, Zonneveld FW, Wiersinga WM. The usefulness of quantitative orbital magnetic resonance imaging in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 2001, 54: 205–9.
Prummel MF, Suttorp-Schulten MS, Wiersinga WM, et al. A new ultrasonographic method to detect disease activity and predict response to immunosuppressive treatment in Graves’ ophthalmopathy. Ophthalmology 1993, 100: 556–61.
Gorman CA, Garrity JA, Fatourechi V, et al. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for Graves’ ophthalmopathy. Ophthalmology 2001, 108: 1523–34.
Frueh BR. Why the NOSPECS classification of Graves’ eye disease should be abandoned, with suggestions for the characterization of the disease. Thyroid 1992, 2: 85–8.
Chen YL, Chang TC, Huang KM, et al. Relationship of eye movement to computed tomographic findings in patients with Graves’ ophthalmopathy. Acta Ophthalmol (Copenh) 1994, 72: 472–7.
Hiromatsu Y, Kojima K, Ishisaka N, et al. Role of magnetic resonance imaging in thyroid-associated ophthalmopathy: its predictive value for therapeutic outcome of immunosuppressive therapy. Thyroid 1992, 2: 299–305.
Durak I, Durak H, Ergin M, Yurekli Y, Kaynak S. Somatostatin receptors in the orbits. Clin Nucl Med 1995, 20: 237–42.
Doumas AS, Krassas GE, Kaltsas T, Pontikides N. Imaging somatostatin receptor activity in patients with active thyroid eye disease using Tc-99m depreotide. Clin Nucl Med 2003, 28: 439–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, TC., Liao, SL. Slow-release lanreotide in Graves’ ophthalmopathy: A double-blind randomized, placebo-controlled clinical trial. J Endocrinol Invest 29, 413–422 (2006). https://doi.org/10.1007/BF03344124
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03344124