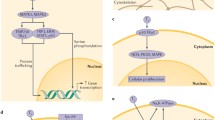
Abstract
Steroids and thyroid hormone are thought primarily to act via binding to hormonespecific nuclear receptor superfamily members. The nuclear ligand-receptor complexes then initiate transcriptional activity. Actions of steroids and iodothyronines that are nongenomic or extranuclear in mechanism have been recognized recently and new insights into such mechanisms are available. Despite their distinct structures and biologic effects, the two families of hormones have similarities in the mechanisms of their nongenomic actions. That is, both steroids and thyroid hormone appear to interact with specific cell surface G protein-coupled receptors and to activate signal transducing kinases such as those involved in the mitogen-activated protein kinase (MAPK) pathway. Much is known about the ability of certain steroids such as estrogen and mineralocorticoids to increase [Ca2+]i acutely and stimulation of the MAPK cascade by L-T4 appears to depend upon a hormone-induced increase in [Ca2+]i via phosphoinositide pathway activation. At least in the case of iodothyronines, hormone activation of the MAPK pathway modulates the cellular activities of certain cytokines and growth factors. One of the two cell surface estrogen receptors (ERs) may be an expression of the same transcript as that for nuclear ER, whereas the mineralocorticoid and progesteronebinding proteins in the plasma membrane appear to be products of genes different from those of nuclear receptors. Iodothyronine structure-activity relationships at the plasma membrane binding site for thyroid hormone suggest that the cell surface receptor for T4 that also binds 3,5,3′-triiodo-L-T3 is different from the nuclear T3 receptor (TR). There are interfaces of nongenomic and genomic mechanisms for both steroids and thyroid hormone. For example, by nongenomic mechanisms, estrogen and thyroid hormone can promote serine phosphorylation, respectively, of nuclear ER and TR. Transcriptional activity of the nuclear receptor proteins can be altered by such phosphorylation.
Similar content being viewed by others
References
Lowe W.L., Jr., Pestell R.G., Madison L.D., Jameson J.L. Mechanisms of hormone action. In: Jameson J.L., Collins F.S. (Eds.), Principles of molecular medicine. Humana Press, Totowa, 1998, p. 419.
Beato M. Gene regulation by steroid hormones. Cell 1989, 56: 335–344.
Chin W.W., Yen P.M. Molecular mechanisms of nuclear thyroid hormone action. In: Braverman L.E. (Ed.), Contemporary endocrinology: Diseases of the thyroid. Humana Press, Totowa, 1997, p. 1.
Zhang J., Lazar M.A. The mechanism of action of thyroid hormones. Annu. Rev. Physiol. 2000, 62: 439–466.
Falkenstein E., Tillmann H.C., Christ M., Feuring M., Wehling M. Multiple actions of steroid hormones — A focus on rapid, nongenomic effects. Pharmacol. Rev. 2000, 52: 513–556.
Davis P.J., Davis F.B. Nongenomic actions of thyroid hormone. In: Braverman L.E. (Ed.), Contemporary endocrinology: Diseases of the thyroid. Humana Press, Totowa, 1997, p. 17.
Incerpi S., Luly P., De Vito P., Farias R.N. Short-term effects of thyroid hormones on the Na/H antiport in L-6 myoblasts: high molecular specificity for 3,3′,5-triiodo-L-thyronine. Endocrinology 1999, 140: 683–689.
Sakaguchi Y., Cui G., Sen L. Acute effects of thyroid hormone on inward rectifier potassium channel currents in guinea pig ventricular myocytes. Endocrinology 1996, 137: 4744–4751.
Huang C.J., Geller H.M., Green W.L., Craelius W. Acute effects of thyroid hormone analogs on sodium currents in neonatal rat myocytes. J. Mol. Cell. Cardiol. 1999, 31: 881–893.
Zhu X.-G., Hanover J., Hager G., Cheng S.-Y. Hormoneinduced translocation of thyroid hormone receptors in living cells visualized using a receptor green fluorescent protein chimera. J. Biol. Chem. 1998, 273: 27058–27063.
Chen Y., Chen P.L., Chen, C.F., Sharp, Z.D., Lee W.H. Thyroid hormone, T3-dependent phosphorylation and translocation of Trip230 from the Golgi complex to the nucleus. Proc. Natl. Acad. Sci. USA 1999, 96: 4443–4448.
Safran M., Farwell A.P., Leonard J.L. Thyroid hormone-dependent redistribution of the 55-kilodalton monomer of protein disulfide isomerase in cultured glial cells. Endocrinology 1992, 131: 2413–2418.
Lin H.-Y., Thacore H.R., Davis F.B., Davis P.J. Potentiation by thyroxine of interferon-gamma-induced antiviral state requires PKA and PKC activities. Am. J. Physiol. 1996, 271: C1256–1261.
Lin H.-Y., Davis F.B., Gordinier J.K., Martino L.J., Davis P.J. Thyroid hormone induces activation of mitogen-activated protein kinase in cultured cells. Am. J. Physiol. 1999, 276: C1014–C1024.
Siegrist-Kaiser C., Juge-Aubry C., Tranter M., Ekenbarger D., Leonard J. Thyroxine-dependent modulation of actin polymerization in cultured astrocytes. A novel, extranuclear action of thyroid hormone. J. Biol. Chem. 1990, 265: 5296–5302.
Davis P.J., Shih A., Lin H.-Y., Martino L.J., Davis F.B. Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J. Biol. Chem. 2000, 275: 38032–38039.
Ebata S., Muto S., Okada K., Nemoto J., Amemiya M., Saito T., et al. Aldosterone activates Na+/H+ exchange in vascular smooth muscle cells by nongenomic and genomic mechanisms. Kidney Int. 56: 1400–1412.
Ashizawa K., McPhie P., Lin K.H., Cheng S.-Y. An in vitro novel mechanism of regulating the activity of pyruvate kinase M2 by thyroid hormone and fructose 1,6-bisphosphate. Biochemistry 1991, 30: 7105–7111.
Arnold S., Goglia F., Kadenbach B. 3,5-Diiodothyronine binds to subunit Va of cytochrome-c oxidase and abolishes the allosteric inhibition of respiration by ATP. Eur. J. Biochem. 1998, 252: 325–330.
Sun Z.-Q., Ojamaa K., Coetzee W., Artman M., Klein I. Effects of thyroid hormone on action potential and repolarizing currents in rat ventricular myocytes. Amer. J. Physiol. 2000, 278: E302–E307.
Giguere A., Fortier S., Beaudry C., Gallo-Payet N., Bellabarba D. Effect of thyroid hormones on G proteins in synaptosomes of chick embryo. Endocrinology 1996, 137: 2558–2564.
Lawrence W.D., Schoenl M., Davis P.J. Stimulation in vitro of rabbit erythrocyte cytosol phospholipid-dependent protein kinase activity. A novel action of thyroid hormone. J. Biol. Chem. 1989, 264: 4766–4768.
Sundquist J., Blas S.D., Hogan J.E., Davis F.B., Davis P.J. The alpha 1-adrenergic receptor in human erythrocyte membranes mediates interaction in vitro of epinephrine and thyroid hormone at the membrane Ca(2+)-ATPase. Cell Signalling 1992, 4: 795–799.
Davis F.B., Moffett M.J., Davis P.J., Al Ogaily M.S., Blas S.D. Inositol phosphates modulate binding of thyroid hormone to human red cell membranes in vitro. J. Clin. Endocrinol. Metab. 1993, 77: 1427–1430.
Davis F.B., Davis P.J., Blas S.D., Gombas D.Z. Inositol phosphates modulate human red blood cell Ca(2+)-adenosine triphosphatase activity in vitro by a guanine nucleotide regulatory protein. Metabolism 1995, 44: 865–868.
Smallwood J.I., Gugi B., Rasmussen H. Regulation of erythrocyte Ca2+ pump activity by protein kinase C. J. Biol. Chem. 1988, 263: 2195–2202.
Galo M.G., Unates L.E., Farias R.N. Effect of membrane fatty acid composition on the action of thyroid hormones on (Ca2+ + Mg2+)-adenosine triphosphatase from rat erythrocyte. J. Biol. Chem. 1981, 256: 7113–7114.
Mylotte K.M., Cody V., Davis P.J., Davis F.B., Blas S.D., Schoenl M. Milrinone and thyroid hormone stimulate myocardial membrane Ca2+-ATPase activity and share structural homologies. Proc. Natl. Acad. Sci. USA 1985, 82: 7974–7978.
Warnick P.R., Davis P.J., Davis F.B., Cody V., Galindo J. Jr., Blas S.D. Rabbit skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase activity: stimulation in vitro by thyroid hormone analogues and bipyridines. Biochem. Biophys. Acta 1993, 1153: 184–190.
Christ M., Meyer C., Sippel K., Wehling M. Rapid aldosterone signaling in vascular smooth muscle cells: involvement of phospholipase C, diacylglycerol and protein kinase C alpha. Biochem. Biophys. Res. Commun. 1995, 213: 123–129.
Lin H.Y., Thacore H.R., Davis F.B., Davis P.J. Thyroid hormone analogues potentiate the antiviral action of interferon- gamma by two mechanisms. J. Cell. Physiol. 1996, 167: 269–276.
Lin H.Y., Yen P.M., Davis F.B., Davis P.J. Protein synthesis-dependent potentiation by thyroxine of antiviral activity of interferon-gamma. Am. J. Physiol. 1997, 273: C1225–C1232.
Lin H.Y., Shih A., Davis F.B., Davis P.J. Thyroid hormone down regulates TGF-a-induced immediate-early gene expression via a PKA-dependent pathway. Proceedings of the 83rd Annual Meeting of the Endocrine Society, June, 2001.
Lin H.Y., Shih A., Davis F.B., Davis P.J. Thyroid hormone promotes the phosphorylation of STAT3 and potentiates the action of epidermal growth factor in cultured cells. Biochem. J. 1999, 338: 427–432.
Schindler C., Darnell J.E. Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 1995, 64: 621–651.
Horvath C.M., Darnell J.E. Jr. The antiviral state induced by alpha interferon and gamma interferon requires transcriptionally active Stat1 protein. J. Virol. 1996, 70: 647–650.
Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995, 270: 1491–1494.
Shih A., Lin H.Y., Davis F.B., Davis P.J. Thyroid hormone promotes serine phosphorylation of p53 by mitogen-activated protein kinase. Biochemistry 2001, 40: 2870–2878.
Puymirat J., Etongue-Mayer P., Dussault J. Thyroid hormones stabilize acetylcholinesterase mRNA in neuro-2A cells that overexpress the beta1 thyroid receptor. J. Biol. Chem. 1995, 270: 30651–30656.
Koenig R.J. Thyroid hormone receptor coactivators and corepressors. Thyroid 1998, 8: 703–713.
Vassy R., Nicolas P., Yin Y.L., Perret G.Y. Nongenomic effect of triiodothyronine on cell surface beta-adrenoceptors in cultured embryonic cardiac myocytes. Proc. Soc. Exp. Biol. Med. 1997, 214: 352–358.
Farwell A.P., DiBenedetto D.J., Leonard J.L. Thyroxine targets different pathways of internalization of type II iodothyronine 5′-deiodinase in astrocytes. J. Biol. Chem. 1993, 268: 5055–5062.
Farwell A.P., Tranter M.P., Leonard J.L. Thyroxine-dependent regulation of integrin-laminin interactions in astrocytes. Endocrinology 1995, 136: 3909–3915.
Zhang X.A., Bontrager A.L., Stipp C.S., Kraeft S.K., Bazzoni G., Chen L.B., et al. Phosphorylation of a conserved integrin alpha 3 QPSXXE motif regulates signaling, motility, and cytoskeletal engagement. Mol. Biol. Cell 2001, 12: 351–365.
Boehme S.A., Sullivan S.K., Crowe P.D., Santos M., Conlon P.J., Sriramarao P., et al. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J. Immunol. 1999, 163: 1611–1618.
Segal J., Ingbar S. Evidence that an increase in cytoplasmic calcium is the initiating event in certain plasma membranemediated responses to 3,5,3′-triiodothyronine in rat thymocytes. Endocrinology 1989, 124: 1949–1955.
Improta-Brears T., Whorton A.R., Codazzi F., York J.D., Meyer T., McDonnell D.P. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc. Natl. Acad. Sci. USA 1999, 96: 4686–4691.
Doolan C.M., Harvey B.J. Modulation of cytosolic protein kinase C and calcium ion activity by steroid hormones in rat distal colon. J. Biol. Chem. 1996, 271: 8763–8767.
Qiu J., Lou L.G., Huang X.Y., Lou S.J., Pei G., Chen Y.Z. Nongenomic mechanisms of glucocorticoid inhibition of nicotine-induced calcium influx in PC12 cells: involvement of protein kinase C. Endocrinology 1998, 139: 5103–5108.
Lieberherr M., Grosse B., Machelon V. Phospholipase C-beta and ovarian sex steroids in pig granulosa cells. J. Cell. Biochem. 1999, 74: 50–60.
Machelon V., Nome F., Grosse B., Lieberherr M. Progesterone triggers rapid transmembrane calcium influx and/or calcium mobilization from endoplasmic reticulum, via a pertussis-insensitive G-protein in granulosa cells in relation to luteinization process. J. Cell. Biochem. 1996, 61: 619–628.
Machelon V., Nome F., Tesarik J. Nongenomic effects of androstenedione on human granulosa luteinizing cells. J. Clin. Endocrinol. Metab. 1998, 83: 263–269.
Harrison D.A., Carr D.W., Meizel S. Involvement of protein kinase A and A kinase anchoring protein in the progesterone- initiated human sperm acrosome reaction. Biol. Reprod. 2000, 62: 811–820.
Richards J.S. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol. Endocrinol. 2001, 15: 209–218.
Christ M., Gunther A., Heck M., Schmidt B.M., Falkenstein E., Wehling M. Aldosterone, not estradiol, is the physiological agonist for rapid increases in cAMP in vascular smooth muscle cells. Circulation 1999, 99: 1485–1491.
Gekle M., Silbernagl S., Oberleithner H. The mineralocorticoid aldosterone activates a proton conductance in cultured kidney cells. Am. J. Physiol. 1997, 273: C1673–C1678.
Sato A., Liu J.P., Funder J.W. Aldosterone rapidly represses protein kinase C activity in neonatal rat cardiomyocytes in vitro. Endocrinology 1997, 138: 3410–3416.
Muto S., Ebata S., Okada K., Saito T., Asano Y. Glucocorticoid modulates Na+/H+ exchange activity in vascular smooth muscle cells by nongenomic and genomic mechanisms. Kidney Int. 2000, 57: 2319–2333.
Lee H.W., Eghbali-Webb M. Estrogen enhances proliferative capacity of cardiac fibroblasts by estrogen receptor- and mitogen-activated protein kinase-dependent pathways. J. Mol. Cell. Cardiol. 1998, 30: 1359–1368.
Bayaa M., Booth R.A., Sheng Y., Liu X.J. The classical progesterone receptor mediates xenopus oocyte maturation through a nongenomic mechanism. Proc. Natl. Acad. Sci. USA 2000, 97: 12607–12612.
Gerdes D., Wehling M., Leube B., Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol. Chem. 1998, 379: 907–911.
Falkenstein E., Schmieding K., Lange A., Meyer C., Gerdes D., Welsch U., et al. Localization of a putative progesterone membrane binding protein in porcine hepatocytes. Cell. Mol. Biol. 1998, 44: 571–578.
Nadal A., Ropero A.B., Laribi O., Maillet M., Fuentes E., Soria B. Nongenomic actions of estrogens and xeno-estrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc. Natl. Acad. Sci. USA 2000, 97: 11603–11608.
Razandi M., Pedram A., Greene G.L., Levin E.R. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999, 13: 307–319.
Goetz R.M., Thatte H.S., Prabhakar P., Cho M.R., Michel T., Golan D.E. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1999, 96: 2788–2793.
Koukouritaki S.B., Margioris A.N., Gravanis A., Hartig R., Stournaras C. Dexamethasone induces rapid actin assembly in human endometrial cells without affecting its synthesis. J. Cell. Biochem. 1997, 65: 492–500.
Koukouritaki S.B., Theodoropoulos P.A., Margioris A.N., Gravanis A., Stournaras C. Dexamethasone alters rapidly actin polymerization dynamics in human endometrial cells: evidence for nongenomic actions involving cAMP turnover. J. Cell. Biochem. 1996, 62: 251–261.
Luconi M., Muratori M., Forti G., Baldi E. Identification and characterization of a novel functional estrogen receptor on human sperm membrane that interferes with progesterone effects. J. Clin. Endocrinol. Metab. 1999, 84: 1670–1678.
Falkenstein E., Norman A.W., Wehling M. Mannheim classification of nongenomically initiated (rapid) steroid action( s). J. Clin. Endocrinol. Metab. 2000, 85: 2072–2075.
Bronson R.A., Peresleni T., Golightly M. Progesterone promotes the acrosome reaction in capacitated human spermatozoa as judged by flow cytometry and CD46 staining. Mol. Hum. Reprod. 1999, 5: 507–512.
Meizel S., Turner K.O. Progesterone acts at the plasma membrane of human sperm. Mol. Cell. Endocrinol. 1991, 77: R1–R5.
Osman R.A., Andria M.L., Jones A.D., Meizel S. Steroid induced exocytosis: the human sperm acrosome reaction. Biochem Biophys. Res. Commun. 1989, 160: 828–833.
Baldi E., Casano R., Falsetti C., Krausz C., Maggi M., Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl. 1991, 12: 323–330.
Turner K.O., Garcia M.A., Meizel S. Progesterone initiation of the human sperm acrosome reaction: the obligatory increase in intracellular calcium is independent of the chloride requirement. Mol. Cell. Endocrinol. 1994, 101: 221–225.
Blackmore P.F., Beebe S.J., Danforth D.R., Alexander N. Progesterone and 17alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J. Biol. Chem. 1990, 265: 1376–1380.
Baldi E., Luconi M., Bonaccorsi L., Maggi M., Francavilla S., Gabriele A., et al. Nongenomic progesterone receptor on human spermatozoa: biochemical aspects and clinical implications. Steroids 1999, 64: 143–148.
Blackmore P.F., Neulen J., Lattanzio F., Beebe S.J. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J. Biol. Chem. 1991, 266: 18655–18659.
Meyer C., Schmid R., Scriba P.C., Wehling M. Purification and partial sequencing of high affinity progesterone-binding site(s) from porcine liver membranes. Eur. J. Biochem. 1996, 239: 726–731.
Falkenstein E., Meyer C., Eisen C., Scriba P.C., Wehling M. Full-length cDNA sequence of a progesterone membranebinding protein from porcine vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1996, 229: 86–89.
Blackmore P.F. Extragenomic actions of progesterone in human sperm and progesterone metabolites in human platelets. Steroids 1999, 64: 149–156.
Selmin O., Lucier G.W., Clark G.C., Tritscher A.M., Vanden Heuvel J.P., et al. Isolation and characterization of a novel gene induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Carcinogenesis 1996, 17: 2609–2615.
Christ M., Eisen C., Aktas J., Theisen K., Wehling M. The inositol-1,4,5-trisphosphate system is involved in rapid effects of aldosterone in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 1993, 77: 1452–1457.
Berridge M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361: 315–325.
Schneider M., Ulsenheimer A., Christ M., Wehling M. Nongenomic effects of aldosterone on intracellular calcium in porcine endothelial cells. Am. J. Physiol. 1997, 272: E616–E620.
Wehling M., Ulsenheimer A., Schneider M., Neylon C., Christ M. Rapid effects of aldosterone on free intracellular calcium in vascular smooth muscle and endothelial cells: subcellular localization of calcium elevations by single cell imaging. Biochem. Biophys. Res. Commun. 1994, 204: 475–481.
Wehling M., Neylon C.B., Fullerton M., Bobik A., Funder J.W. Nongenomic effects of aldosterone on intracellular Ca2+ in vascular smooth muscle cells. Circ. Res. 1995, 76: 973–979.
Doolan C.M., Harvey B.J. Rapid effects of steroid hormones on free intracellular calcium in T84 colonic epithelial cells. Am. J. Physiol. 1996, 271: C1935–C1941.
Haseroth K., Gerdes D., Berger S., Feuring M., Gunther A., Herbst C., et al. Rapid nongenomic effects of aldosterone in mineralocorticoid-receptor-knockout mice. Biochem. Biophys. Res. Commun. 1999, 266: 257–261.
Karim Z.G., Chambrey R., Chalumeau C., Defontaine N., Warnock D.G., Paillard M., et al. Regulation by PKC isoforms of Na(+)/H(+) exchanger in luminal membrane vesicles isolated from cortical tubules. Am. J. Physiol. 1999, 277: F773–F778.
Moor A.N., Fliegel L. Protein kinase-mediated regulation of the Na+/H+ exchanger in the rat myocardium by mitogenactivated protein kinase-dependent pathways. J. Biol. Chem. 1999, 274: 22985–22992.
Sartori M., Ceolotto G., Semplicini A. MAPKinase and regulation of the sodium-proton exchanger in human red blood cell. Biochem. Biophys. Acta 1999, 1421: 140–148.
Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 2000, 21: 90–113.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davis, P.J., Tillmann, H.C., Davis, F.B. et al. Comparison of the mechanisms of nongenomic actions of thyroid hormone and steroid hormones. J Endocrinol Invest 25, 377–388 (2002). https://doi.org/10.1007/BF03344022
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03344022