Abstract
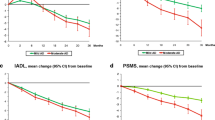
Background and aims: Recently, the Italian Ministry of Health started a national project (CRONOS project), aiming at assessing how a multi-level therapeutic approach — including 2-year free-of-charge treatment with cholinesterase inhibitors (ChE-I), pharmacologic and non-pharmacologic management of behavioral disorders, periodic multi-dimensional assessment, and informal caregivers’ counseling — performs in subjects with mild-to-moderate Alzheimer’s disease (AD). Five hundred and three Alzheimer Evaluation Units (AEUs) were instituted for this purpose all over Italy. In this paper we present the results of this approach in a large population of AD subjects followed for 36 weeks by 14 AEUs in Eastern Lombardy, Italy. Methods: The project lasted for two years (September 2000-September 2002). Subjects eligible for the CRONOS project had a diagnosis of probable AD, a Mini Mental State Examination (MMSE) score at baseline ranging from 10 to 26, and onset of cognitive disorders between 40 and 90 years of age. Periodic clinical and multi-dimensional assessments, including MMSE, Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) were made at 12 and 36 weeks; ChE-I doses, psychotropic and antidepressant drugs were also re-assessed at all clinical examinations. Caregivers were instructed about dementia and drug-related problems. Results: Of the 808 subjects who completed the 36-week follow-up, 441 were naïves (i.e., never previously treated with ChE-I drugs) and 367 non-naïves. At 12 weeks, both naïves (mean variation from baseline= 0.8 points) and non-naïves (mean variation from base-line= 0.5 points) improved their MMSE scores, while at 36 weeks only naïves improved (mean variation from baseline= 0.1) and non-naïves decreased (mean variation from baseline= −1.2). The IADL and ADL scores progressively and mildly declined from baseline to the 36th week (ADL, mean variation from baseline= −0.5 for naïves, −0.3 for non-naïves; IADL= −0.7 for naïves, mean variation from baseline= −0.4). However, when the MMSE, ADL and IADL variations were controlled for age, sex and education, no significant time effect was found (MMSE, Wilks’ lambda p=0.34; ADL, Wilks’ lambda p=0.25; IADL, Wilks’ lambda p=0.3, respectively). These patterns were apparently unrelated to ChE-I doses. Neuroleptic use doubled in naïves and antidepressants increased in both groups. Conclusions: This multi-level therapeutic approach seems to slow down progression in cognitive and functional performance, in both naïve and non-naïve subjects. The possibility of recurrent examinations by specialized physicians, accurate, close management of psychotropic drugs, and informal counseling to caregivers probably aid in achieving such results in a “real world” population of AD elderly subjects living at home. Future studies are needed to assess whether a multi-level therapeutic approach including higher ChE-I dose may perform better in these subjects.
Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, D.C.: American Psychiatric Association, 1994.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34: 939–44.
Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98.
Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970; 10: 20–30.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–86.
O’Brien JT, Ballard CG. Drugs for Alzheimer’s disease. BMJ 2001; 323: 123–4.
Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology 1998; 50: 136–45.
Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease for the ENA 713 B352 Study Group. Int J Geriatr Psychopharmacol 1998; 1: 55–65.
Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomized controlled trial. Galantamine International-1 Study Group. BMJ 2000; 321: 1445–9.
Rogers SL, Doody RS, Pratt RD, Ieni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: final analysis of a US multicentre open-label study. Eur Neuropsychopharmacol 2000; 10:195–203.
Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 2000; 54: 2261–8.
Farlow M, Anand R, Messina J Jr, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol 2000; 44: 236–41.
Winblad B, Engedal K, Soininen H, et al., Donepezil Nordic Study Group. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001; 57: 489–95.
Trinh NH, Hoblyn J, Mohanty S. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer Disease. A meta-analysis. JAMA 2003; 289: 210–6.
Magni E, Binetti G, Bianchetti A, Trabucchi M. Risk of mortality and institutionalization in demented patients with delusions. J Geriatr Psychiatry Neurol 1996; 3: 123–6.
Rainer MK, Masching AJ, Ertl MG, Kraxberger E, Haushofer M. Effect of risperidone on behavioral and psychological symptoms and cognitive function in dementia. J Clin Psychiatry 2001; 62: 894–900.
Sands LP, Yaffe K, Covinsky K, et al. Cognitive screening predicts magnitude of functional recovery from admission to discharge in hospitalized elders. J Gerontol 2003; 58: 37–45.
Bianchetti A, Frisoni GB, Trabucchi M. Do old age psychiatrists miss physical illness? Int J Geriatr Psychiatry 1993; 8: 356–7.
Sabir M, Pillemer K, Suitor J, Patterson M. Predictors of successful relationships in a peer support program for Alzheimer’s caregivers. Am J Alzheimers Dis Other Demen 2003; 18: 115–22.
Volicer L, Hurley AC. Management of behavioral symptoms in progressive degenerative dementias. J Gerontol 2003; 58: M837–45.
Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56: 1154–66.
Bianchetti A, Padovani A, Trabucchi M. Outcomes of Alzheimer’s disease treatment: the Italian CRONOS project. Int J Geriatr Psychiatry 2003; 18: 87–8.
Cummings JL. Use of cholinesterase inhibitors in clinical practice: evidence based recommendations. Am J Geriatr Psychiatry 2003; 11: 131–45.
Tunis, SR, Stryer DB, Clancy CM. Practical clinical trials. Increasing the value of clinical research for decision making in clinical and healthy policy. JAMA 2003; 290: 1624–32.
Frisoni GB. Treatment of Alzheimer’s disease with acetylcholinesterase inhibitors: bridging the gap between evidence and practice. J Neurol 2001; 248: 551–7.
Albert SM, Sano M, Marder K, et al. Participation in clinical trials and long-term outcomes in Alzheimer’s disease. Neurology 1997; 49: 38–43.
Schneider IS, Olin JT, Lyness SA, et al. Eligibility of Alzheimer’s disease clinic patients for clinical trials. J Am Geriatr Soc 1997; 43: 923–8.
Bayer T. Commentary: Another piece of the Alzheimer’s jigsaw. BMJ 1999; 318: 633–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellelli, G., Lucchi, E., Minicuci, N. et al. Results of a multi-level therapeutic approach for Alzheimer’s disease subjects in the “real world” (CRONOS project): a 36-week follow-up study. Aging Clin Exp Res 17, 54–61 (2005). https://doi.org/10.1007/BF03337721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03337721