Abstract
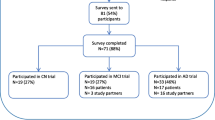
Background: Because of the growing value attributed to informed consent, competence assessment has become an important task for physicians and researchers, particularly when treatment and research involve persons who may be cognitively impaired, such as those with Alzheimer’s dementia. Methods: We developed and validated a 12-item questionnaire to assess the understanding of information about clinical trials by research subjects (score 0 to 24). The 12 questions were selected from a larger pool of 16 through internal consistency validity testing. We used the instrument in a pilot study involving 42 patients with mild to moderate Alzheimer’s disease who had been asked to take part in two randomized clinical trials, and 21 caregivers. Results: Patients with Alzheimer’s disease had poor understanding (mean global score on questionnaire: 6.1±3.5). Unlike patients, caregivers understand the key elements of the clinical trials (questionnaire mean global score: 21.5±2.3; p for difference with patients <0.0001). In the group of patients, the score on the questionnaire correlated weakly with the Mini Mental State Exam (r=0.351; p=0.023), but more strongly with years of education in the group of caregivers (r=0.548; p=0.010) and age (r=-0.540; p=0.011). Conclusions: Patients with Alzheimer’s disease of mild to moderate severity show poor understanding of the design, risks and benefits of clinical trials. Enrolment of these patients in clinical trials must be accompanied by adequate measures for patient protection.
Similar content being viewed by others
Reference
American Psychiatrie Association. Guidelines for assessing decision-making capacities of potential research subjects with cognitive impairment. Am J Psychiatry 1998; 155: 1649–50.
Defanti CA, Tiezzi A, Gasparini M et al., for the Bioethics and Palliative Care in Neurology Study Group of the Italian Society of Neurology. Ethical questions in the treatment of subjects with dementia. Part I. Respecting autonomy: awareness, competence and behavioural disorders. Neurol Sci 2007; 28: 216–31.
Dunn LB, Nowrangi MA, Palmer BW, Jeste DV, Saks ER. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry 2006; 163: 1323–34.
Kim SYH, Karlawish JHT, Caine ED. Current state of research on decision-making competence of cognitively impaired elderly persons. Am J Geriatr Psychiatry 2002; 10: 151–65.
Sturman ED. The capacity to consent to treatment and research: a review of standardized assessment tools. Clin Psychol Rev 2005; 25: 954–74.
AGS Ethics Committee. Informed consent for research on human subjects with dementia. J Am Geriatr Soc 1998; 46: 1308–10.
Brodaty H, Dresser R, Eisner M et al. Alzheimer’s Disease International and International Working Group for harmonization of dementia drug guidelines for research involving human subjects with dementia. Alzheimer Dis Assoc Disord 1999; 13: 71–9.
Glass KC. Refining definitions and devising instruments: two decades of assessing mental competence. Int J Law Psychiatry 1997; 20: 5–33.
High DM, Whitehouse PJ, Post SG, Berg L. Guidelines for addressing ethical and legal issues in Alzheimer Disease research: a position paper. Alzheimer Dis Assoc Disord 1994; 8 (Suppl. 4): 66–74.
Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research. A systematic review. JAMA 2004; 292: 1593–601.
Appelbaum PS, Grisso T. MacArthur Competence Assessment Tool for Clinical Research. Sarasota, FL: Professional Resource Press, 2001.
Jeste DV, Palmer BW, Appelbaum PS et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry 2007; 64: 966–74.
Karlawish JHT, Casarett DJ, James BD. Alzheimer’s disease patients’ and caregivers’ capacity, competency, and reasons to enroll in an early-phase Alzheimer’s disease clinical trial. J Am Geriatr Soc 2002; 50: 2019–24.
Kim SYH, Caine ED, Currier GW, Leibovici A, Ryan JM. Assessing the competence of persons with Alzheimer’s disease in providing informed consent for participation in research. Am J Psychiatry 2001; 158: 712–7.
Palmer BW, Dunn LB, Appelbaum PS et al. Assessment of capacity to consent to research among older persons with schizophrenia, Alzheimer disease, or diabetes mellitus. Arch Gen Psychiatry 2005; 62: 726–33.
Karlawish J, Kim SYH, Knopman D, van Dyck CH, James BD, Marson D. Interpreting the clinical significance of capacity scores for informed consent in Alzheimer disease clinical trials. Am J Geriatr Psychiatry 2008; 16: 568–74.
Marson DC, Earnst KS, Jamil F, Bartolucci A, Harrell LE. Consistency of physicians’s legal standards and personal judgments of competency in patients with Alzheimer’s disease. J Am Geriatr Soc 2000; 48: 911–8.
Bean G, Nishisato S, Rector NA, Glancy G. The psychometric properties of the Competency Interview Schedule. Can J Psychiatry 1994; 39: 368–76.
Bean G, Nishisato S, Rector NA, Glancy G. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry 1996; 41: 85–92.
Grisso T, Appelbaum PS. MacArthur Competence Assessment tool for treatment. Sarasota, FL: Professional Resource Press, 1998.
Janofsky JS, McCarthy RJ, Folstein MF. The Hopkins Competency Assessment Test: a brief method for evaluating patients’ capacity to give informed consent. Hosp Community Psychiatry 1992; 43: 132–6.
Marson DC, Ingram KK, Cody HA, Harrell LE. Assessing the competency of patients with Alzheimer’s disease under different legal standards. A prototype instrument. Arch Neurol 1995; 52: 949–54.
Miller CK, O’Donnell DC, Searight R, Barbarash RA. The Deaconess informed consent comprehension test: an assessment tool for clinical research subjects. Pharmacotherapy 1996; 16: 872–8.
Sachs GA, Stocking CB, Stern R, Cox DM, Hougham G, Sachs RS. Ethical aspects of dementia research: informed consent and proxy consent. Clin Res 1994; 42: 403–12.
Saks ER, Dunn LB, Marshall BJ, Nayak GV, Golshan S, Jeste DV. The California Scale of Appreciation. A new instrument to measure the appreciation component of capacity to consent to research. Am J Geriatr Psychiatry 2002; 10: 166–74.
Schmand B, Gouwenberg B, Smit JH, Jonker C. Assessment of mental competency in community-dwelling elderly. Alzheimer Dis Assoc Disord 1999; 13: 80–7.
Tomoda A, Yasumiya R, Sumiyama T, et al. Validity and reliability of Structured Interview for Competency Incompetency Assessment Testing and Ranking Inventory. J Clin Psychol 1997; 53: 443–50.
Wirshing DA, Wirshing WC, Marder SR, Liberman RP, Mintz J. Informed consent: assessment of comprehension. Am J Psychiatry 1998; 155: 1508–11.
ICH, Good Clinical Practice. Guidelines, 1996.
McKahnn G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34: 939–44.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 1975; 12: 189–98.
Pucci E, Belardinelli N, Borsetti G, Rodriguez D, Signorino M. Information and competency for consent to pharmacologic clinical trials in Alzheimer disease: an empirical analysis in patients and family caregivers. Alzheimer Dis Assoc Disord 2001; 15: 146–54.
Kim SYH, Caine ED. Utility and limits of the Mini Mental State Examination in evaluating consent capacity in Alzheimer’s disease. Psychiatr Serv 2002; 53: 1322–4.
Marson DC, Schmitt FA, Ingram KK, Harrell LE. Determining the competency of Alzheimer patients to consent to treatment and research. Alzheimer Dis Assoc Disord 1994; 8 (Suppl. 4): 5–18.
Vellinga A, Smit JH, Van Leeuwen E, Van Tilburg W, Jonker C. Decision-making capacity of elderly patients assessed through the vignette method: imagination or reality? Aging Ment Health 2005; 9: 40–8.
Elliott C. Mentally disabled and mentally ill persons. Research issues. In Post SG, ed. Encyclopedia of Bioethics. 3rd ed., New York: Macmillan, 2004: 1825–30.
National Bioethics Advisory Commission. Research Involving Persons With Mental Disorders That May Affect Decision-making Capacity. Rockville, Maryland, 1998.
Warner J, McCarney R, Griffin M, Hill K, Fisher P. Participation in dementia research: rates and correlates of capacity to give informed consent. J Med Ethics 2008; 34: 167–70.
Buckles VD, Powlishta KK, Palmer JL et al. Understanding of informed consent by demented individuals. Neurology 2003; 61: 1662–6.
Geiselmann B. Helmehen H. Demented subjects’ competence to consent to participate in field studies: the Berlin Ageing Study. Med Law 1994; 13: 177–84.
Alzheimer’s Association. Research consent for cognitively impaired adults. Recommendations for Institutional Review Boards and Investigators. Alzheimer Dis Assoc Disord 2004; 18: 171–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porteri, C., Andreatta, C., Anglani, L. et al. Understanding information on clinical trials by persons with Alzheimer’s dementia. A pilot study. Aging Clin Exp Res 21, 158–166 (2009). https://doi.org/10.1007/BF03325224
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03325224