Abstract
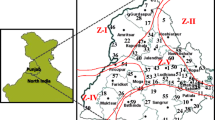
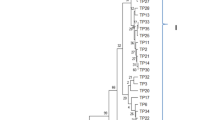
Rice sheath blight fungus Rhizoctonia solani has a wide host range and is highly variable in pathogenecity, sclerotial production and cultural characteristics. In India, breeding for sheath blight resistant cultivars has been a priority area of research. However, lack of adequate information about the genetic variability of the fungal populations occurring in India, non-availability of appropriate markers and the non-availability of resistant donors are some of the limiting factors to achieve this objective. To assess the genetic variability in sheath blight fungus, 18 isolates collected from different rice growing regions of India were analyzed by using random amplified polymorphic DNA (RAPD) markers.The similarity values of RAPD profiles ranged from 0.41 to 0.85 with an average of 0.66 among all the isolates. The percentage polymorphism detected per primer varied from 79.2 to 100%. All the primers could be used to fingerprint the individual isolates. The cluster analysis using unweighted paired group method with arithmetic averages could distinguish between R. solani isolates as well as the virulent and avirulent isolates on rice.
Similar content being viewed by others
References
Ou SH, In Rice diseases, Commonwealth Mycological Institute, Kew (1985) p 380.
Sneh B, Burpee L, & Ogoshi A, In Identification of Rhizoctonia species, The American Phytopatholgical Society, APS Press, St Paul (1991) p 135.
Bentley S, Pegg KG, Moore NY & Davis RD, Phytopathology, 88 (1998) 1283.
Kolmer JA & Liu JQ, Phytopathology, 90 (2000) 427.
Duncan S., Barton JE & O’Brien PA, Mycol Res, 97 (1993) 1075.
Yang HA, Sivasithamparm K, Barton JE & O’Brien, Plant Pathol, 44 (1995) 811.
Yang J, Kharbanda PD, Wang H & McDrew DW, Plant Dis, 80 (1996) 513.
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA & Tingey S, Nucleic Acids Res, 18 (1990) 6531.
Liu ZL & Sinclair JB, Phytopathology, 82 (1992) 778.
Balali GR, Neate SM, Scott ES & Whisson DL, In Intl Symp on Rhizoctonia, June 27-30, Norrwikerhout (1994) p 1.
Vilgalys R & Cubeta MA, Ann Rev of Phytopathol, 32 (1994) 135.
Salazar O, Schneider JHM, Julian MC, Keijer J & Rubio V, Mycologia, 91 (1999) 459.
Cubeta MA & Vilgalys R, Phytopathology, 87 (1997) 480.
Zuber M & Manibhushan Rao K, Canadian J Microbiol, 28 (1982) 762.
Reddy CS, Indian Phytopathological Society - Golden Jubilee International Conference, New Delhi, Nov 10-15, (1997) p 424.
Bhaktavatsalam G, Satyanarayana K, Reddy APK & John VT, Int Rice Res News, 13 (1978) 9.
IRRI Annual Report, IRRI, Manila, Philippines (1986).
Bennett J & Nair S, In Genome analysis of plants, pests and pathogens, ICGEB, New Delhi, (1993) p 141.
Sokal RR & Michener CD, Univ Kansas Sci Bull, 38 (1958) 1409.
Rohlf FJ, NTSYS-PC Version 2.0. Exeter Software, Setauket, New York (1993).
Yap I & Nelson RJ, IRRI discussion paper series No. 14, International Rice Research Institute, Manila, Philippines (1996).
Balali GR, Whisson DL, Scott ES & Neate SM, Mycol Res, 100 (1996) 467.
Milgroom MG, Ann Rev Phytopathol, 25 (1996) 383.
Banniza S, Rutherford MA, Bridge PK, Holderness M & Mordue JE, In Proc Intl Conf Crop Protection: Pests and Diseases Vol 1, Brighton, November 18-21, (1996) p 399.
Kuninaga S & Yokosawa R, Ann Phytopathol Soc Jpn, 48 (1982) 659.
Kuninaga S, Yokosawa R & Ogoshi A, Ann Phytopathol Soc Jpn. 45 (1979) 201.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neeraja, C.N., Vijayabhanu, N., Shenoy, V.V. et al. RAPD Analysis of Indian Isolates of Rice Sheath Blight Fungus Rhizoctonia solani . J. Plant Biochem. Biotechnol. 11, 43–48 (2002). https://doi.org/10.1007/BF03263133
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03263133