Abstract
Background: Examination of clinical data routinely recorded in general practice provides significant opportunities for identifying and quantifying medicine-related adverse events not captured by spontaneous adverse reaction reporting systems. Robust pharmacovigilance methods for detecting and monitoring adverse events due to treatment with new and existing medicines are required to estimate the true extent of adverse events experienced by primary care patients.
Objectives: The aim of the study was to examine evidence of adverse events contained in general practice electronic records and to study observed events related to selective serotonin reuptake inhibitors (SSRIs) as an example of drug-specific pharmaceutical surveillance achievable with these data.
Methods: Electronic clinical records for a cohort of 338 931 patients consulting from 2002 to 2007 were extracted from the patient management systems of 30 primary care clinics in New Zealand. Medical warnings files, prescription records and free text consultation notes were used to identify physician-recorded treatment cautions, including adverse events and medicines they were associated with. A structured chronological analysis of prescriptions, consultation notes and adverse events relating to patients prescribed the SSRI citalopram was undertaken, and included investigating reasons for switching treatment to another SSRI (fluoxetine or paroxetine) as a method for detecting evidence of drug safety signals. We compared the number of adverse events identified for patients at one practice with the number spontaneously reported to New Zealand’s Centre for Adverse Reactions Monitoring (CARM).
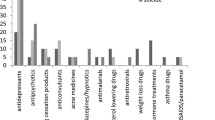
Results: During the 6-year study period, 173 478 patients received 4811 561 prescriptions. There were 37 397 allergies, adverse events and other warnings recorded for 24994 patients (7.4%); adverse events relating to 65 different types of drug were reported. Medicines most frequently implicated in adverse event reports were antibacterials, analgesics, antihypertensive medicines, lipid-modifying agents and skin preparations. Citalopram was prescribed for 5612 patients, and 701 adverse events relating to citalopram were identified in the electronic health records of 473 (8.4%) patients. A total of 713 (12.7%) patients changed treatment from citalopram to another SSRI, and 164 reasons for the switch were identified: suspected adverse drug effects for 129 (78.7%), lack of effect for 29 (17.7%) and patient preference for 6 (3.7%). The most common adverse events preceding the switch were anxiety, nausea and headaches. Of the 725 adverse events and medical warnings recorded at one practice, 21 (2.9%) were spontaneously reported to the CARM.
Conclusions: Routinely recorded general practice data provide a wealth of opportunities for monitoring drug safety signals and for other patient safety issues. Medical warning records and consultation notes contain a wealth of information on adverse events but structured search methodologies are often required to identify these.




Similar content being viewed by others
References
Harmark L, van Grootheest AC. Pharmacovigilance: methods, recent developments and future perpectives. Eur J Clin Pharmacol 2008; 64: 743–52
Almenoff JS. Innovations for the future of pharmacovigilance. Drug Saf 2007; 30(7): 631–3
Wilson AM, Thabane L, Holbrook A. Application of data mining techniques in pharmacovigilance. Br J Clin Pharmacol 2003; 57(2): 127–34
Hauben M. Signal detection in the pharmaceutical industry: integrating clinical and computer approaches. Drug Saf 2007; 30(7): 627–30
Bate A, Lindquist M, Edwards IR. The application of knowledge discovery in databases to post-marketing drug safety: example of the WHO database. Fundam Clin Pharmacol 2008; 22(2): 127–40
Bate A, Lindquist M, Orre R, et al. Data-mining analyses of pharmacovigilance signals in relation to relevant comparison drugs. Eur J Clin Pharmacol 2002; 58(7): 483–90
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf 2006; 29(5): 385–96
Runciman WB, Roughead EE, Semple SJ, et al. Adverse drug events and medication errors in Australia. Int J Qual Health Care 2003; 15 Suppl. 1: i49–59
Platt R, Wilson M, Chan KA, et al. The new sentinel network: improving the evidence of medical-product safety. N Engl J Med 2009; 361(7): 645–7
Behrman RE, Benner JS, Brown JS, et al. Developing the sentinel system: a national resource for evidence development. N Engl J Med 2001; 364(6): 498–9
Stang PE, Ryan PB, Racoosin JA, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med 2010; 153: 600–6
Reisinger SJ, Ryan PB, O’Hara DJ, et al. Development and evaluation of a common data model enabling active drug surveillance using disparate healthcare databases. J Am Med Inform Assoc 2010; 17: 652–62
Coloma PM, Schuemie MJ, Trifiro G, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR project. Pharmacoepidemiol Drug Saf 2010; 20: 1–11
Associate Minister of Health, Minister of Health. Actioning medicines New Zealand. Wellington: Ministry of Health, 2010 [online]. Available from URL: http://www.health.govt.nz/publication/actioning-medicines-new-zealand [Accessed 2012 Jul 25]
Kunac DL, Harrison-Woolrych M, Tatley MV. Pharma covigilance in New Zealand: the role of the New Zealand Pharmacovigilance Centre in facilitating safer medicines use. N Z Med J 2008; 121(1283): 76–89
Classen DC, Pestotnik SL, Evans RS, et al. Computerized surveillance of adverse drug events in hospital patients. JAMA 1991; 266: 2847–51
Zha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc 1998; 5(3): 305–13
Bates DW, Evans RS, Murff H, et al. Detecting adverse events using information technology. J Am Med Inform Assoc 2003; 10(2): 115–28
Melton GB, Hripcsak G. Automated detection of adverse events using natural language processing of discharge summaries. J Am Med Inform Assoc 2005; 12(4): 448–57
Honigman B, Lee J, Rothschild J, et al. Using computerized data to identify adverse drug events in outpatients. J Am Med Inform Assoc 2001; 8(3): 254–66
Seger AC, Gandhi TK, Hope C, et al. Development of a computerized adverse drug event (ADE) monitor in the outpatient setting. In: Henriksen K, Battles JB, Marks ES, et al., editors. Advances in patient safety: from research to implementation. Rockville (MD): Agency for Healthcare Research and Quality, 2005; 2: 173–83
Wood L, Martinez C. The general practice research database: role in pharmacovigilance. Drug Saf 2004; 27(12): 871–81
Johansson S, Wallander M, de Abajo FJ, et al. Prospective drug safety monitoring using the UK primary-care general practice research database: theoretical framework, feasibility analysis and extrapolation of future scenarios. Drug Saf 2010;33(3):223–32
Selak V, Rafter N, Parag V, et al. Cardiovascular treatment gaps: closing, but slowly. N Z Med J 2009; 122(1293): 3564
Didham R, Dovey S, Reith D. Characteristics of general practitioner consultations prior to suicide: a nested case-control study in New Zealand. N Z Med J 2006; 119(1247): U2358
Johansson M, Hall J, Reith D, et al. Trends in the use of inhaled corticosteroids for childhood asthma in New Zealand. Eur J Clin Pharmacol 2003; 59(5–6): 483–7
Pethica BD, Penrose A, MacKenzie D, et al. Comparison of potency of inhaled beclomethasone and budesonide in New Zealand: retrospective study of computerised general practice records. BMJ 1998; 317(7164): 986–90
Warrer P, Hansen EH, Juhl-Jensen L, et al. Using text-mining techniques in electronic patient records to identify ADRs from medicine use. Br J Clin Pharmacol 2012; 73(5): 674–84
Trifiro G, Pariente A, Coloma PM, et al. Data mining on electronic health record databases for signal detection in pharmacovigilance: which events to monitor? Pharmaco epidemiol Drug Saf 2009; 18: 1176–84
Stricker BH, Psaty BM. Detection, verification and quantification of adverse drug reactions? BMJ 2004; 329: 44–7
Dovey S, Loh LW, Cunningham WK. Leveraging information from New Zealand statistical data: a first step to wisdom in transforming unmet need for general practice services. N Z Med J 2011; 124(1334): 15–7
Acknowledgements
Funding for this research was initially provided by a Medsafe/Health Research Council (HRC) of New Zealand product vigilance feasibility study grant (Medsafe/HRC Feasibility Study PV-18). The authors’ research and report were conducted independently of these study sponsors.
We would like to thank the doctors and practice nurses of the general practices that contributed data for this research.
Ethical approval for this research was granted by the New Zealand Multi-Region Ethics Committee, Ministry of Health, Level 2, 1–3 The Terrace, P.O. Box 5013, Wellington, New Zealand (MEC/08/04/EXP).
The authors declare no competing interests with regard to this study.
All authors were involved in the conception and design of the study. Andrew Tomlin and Murray Tilyard were responsible for the acquisition of study data. Andrew Tomlin, David Reith and Susan Dovey conducted the analysis and drafted the article. Murray Tilyard provided critical revision of the content of the article. All authors approved the final version to be published. No other person may be considered an author of this research paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomlin, A., Reith, D., Dovey, S. et al. Methods for Retrospective Detection of Drug Safety Signals and Adverse Events in Electronic General Practice Records. Drug Saf 35, 733–743 (2012). https://doi.org/10.1007/BF03261970
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261970