Abstract
Background: Previous studies have reported telaprevir is effective for chronic hepatitis C virus infection, but the safety of a telaprevir-based regimen remains uncertain.
Objective: A meta-analysis was performed to assess the safety of the addition of telaprevir to a standard regimen of pegylated interferon (peginterferon) plus ribavirin (combination telaprevir with peginterferon plus ribavirin, the TPR group) compared with the standard regimen group (peginterferon plus ribavirin, the PR group).
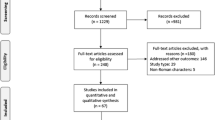
Methods and Results: Seven randomized controlled trials involving a total of 2808 patients were included in the meta-analysis. The addition of telaprevir to the standard regimen was associated with a significantly increased risk of serious adverse events compared with the standard PR group (relative risk [RR]=1.56; 95% confidence interval [CI] 1.21, 2.03; p = 0.0007; I2 = 0%). Telaprevir was also associated with increased risk of treatment discontinuation (RR = 2.10; 95% CI 1.56, 2.83; p<0.0001; I2=42%). In addition, telaprevir was more likely to cause nausea (RR= 1.39; p < 0.0001), diarrhoea (RR = 1.32; p = 0.004), pruritus (RR = 1.56; p = 0.0006), rash (RR = 1.60; p < 0.0001) and anaemia (RR = 1.55; p = 0.007). There was no difference in the other kinds of adverse events between the two groups. Sensitivity analysis further validated the credibility of the above outcomes.
Conclusion: Our meta-analysis raises safety concerns about the potential for an increased risk of serious adverse events associated with the use of telaprevir among patients with chronic hepatitis C virus infection, and cautious use of telaprevir is warranted.






Similar content being viewed by others
References
Lin C, Lin K, Luong YP, et al. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: structural analysis indicates different resistance mechanisms. J Biol Chem. 2004 Apr 23; 279(17): 17508–14
Smith LS, Nelson M, Naik S, et al. Telaprevir: an NS3/4A protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011 May; 45(5): 639–48
Hofmann WP, Zeuzem S. A new standard of care for the treatment of chronic HCV infection. Nat Rev Gastroenterol Hepatol 2011 May; 8(5): 257–64
Pawlotsky JM. The results of Phase III clinical trials with telaprevir and boceprevir presented at the Liver Meeting 2010: a new standard of care for hepatitis C virus genotype 1 infection, but with issues still pending. Gastroenterology 2011 Mar; 140(3): 746–54
Burney T, Dusheiko G. Overview of the PROVE studies evaluating the use of telaprevir in chronic hepatitis C genotype 1 patients. Expert Rev Anti Infect Ther 2011 Feb; 9(2): 151–60
McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009 Apr 30; 360(18): 1827–38
Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011 Jun 23; 364(25): 2405–16
Hezode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009 Apr 30; 360(18): 1839–50
Petitti DB. Meta-analysis, decision analysis, and cost effectiveness analysis: methods for quantitative synthesis in medicine(2nded). New York, NY: Oxford University Press; 2000
Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011 Jun 23; 364(25): 2417–28
Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med 2011 Sep 15; 365(11): 1014–24
McHutchison JG, Manns MP, Muir AJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med 2010 Apr 8; 362(14): 1292–303
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996 Feb; 17(1): 1–12
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959 Apr; 22(4): 719–48
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986 Sep; 7(3): 177–88
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003 Sep 6; 327(7414): 557–60
Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10(1): 101–29
Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull 1999; 8: 15–7
Kumada H, Toyota J, Okanoue T, et al. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol 2012 Jan; 56(1): 78–84
Marcellin P, Forns X, Goeser T, et al. Telaprevir is effective given every 8 or 12 hours with ribavirin and peginterferon alfa-2a or -2b to patients with chronic hepatitis C. Gastroenterology 2011 Feb; 140(2): 459–8 e1; quiz e14
Foster GR, Hezode C, Bronowicki JP, et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology 2011 Sep; 141(3): 881–9 e1
Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001 Jul 5; 345(1): 41–52
Blonski W, Reddy KR. Hepatitis C virus infection and hepato-cellular carcinoma. Clin Liver Dis 2008 Aug; 12(3): 661–74, x
Asselah T. Realize the advance in HCV treatment, but remain cautious. J Hepatol 2011 Dec; 55(6): 1457–60
Cacoub P, Bourliere M, Lubbe J, et al. Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol 2012 Feb; 56(2): 455–63
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011 Apr; 64(4): 383–94
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008 Apr 26; 336(7650): 924–6
Ramachandran P, Fraser A, Agarwal K, et al. UK consensus guidelines for the use of the protease inhibitors boceprevir and telaprevir in genotype 1 chronic hepatitis C infected patients. Aliment Pharmacol Ther 2012 Mar; 35(6): 647–62
Liapakis A, Jacobson I. Telaprevir user’s guide. Clin Liver Dis 2011 Aug; 15(3): 555–71
Soriano V, Vispo E, Poveda E, et al. Treatment failure with new hepatitis C drugs. Expert Opin Pharmacother 2012 Feb; 13(3): 313–23
Kwo PY. Phase III results in Genotype 1 naive patients: predictors of response with boceprevir and telaprevir combined with pegylated interferon and ribavirin. Liver Int 2012 Feb; 32 Suppl. 1: 39–43
Ahlenstiel G, Booth DR, George J. IL28B in hepatitis C virus infection: translating pharmacogenomics into clinical practice. J Gastroenterol 2010 Sep; 45(9): 903–10
Akuta N, Suzuki F, Hirakawa M, et al. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology 2010 Aug; 52(2): 421–9
Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA 2007 Feb 21; 297(7): 724–32
Imhof I, Simmonds P. Genotype differences in susceptibility and resistance development of hepatitis C virus to protease inhibitors telaprevir (VX-950) and danoprevir (ITMN-191). Hepatology 2011 Apr; 53(4): 1090–9
Acknowledgements
The authors have no conflicts of interest that are directly relevant to this study. No external funding was received for the conduct of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, H., Li, H., Zhou, X. et al. Safety of Telaprevir for Chronic Hepatitis C Virus Infection. Clin Drug Investig 32, 665–672 (2012). https://doi.org/10.1007/BF03261920
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261920