Summary
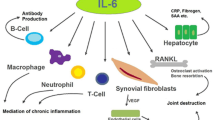
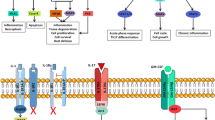
During the last decade many studies have been published pointing to the central role of pro-inflammatory cytokines in the pathogenesis of the inflammatory process in rheumatoid arthritis. Clinical trials targeting several different cytokines have been performed and have yielded positive results, with anti-tumour necrosis factor-α (TNFα) trials being the most promising.
Dysregulation of cytokine production, TNFα in particular, might be an essential step in the cascade of mediators regulating inflammation. Thus, this review examines the effects of current interventions targeted at cytokines. In addition, the available information on the effect of established antirheumatic drugs on cytokine production is examined, and promising future prospects such as transcription inhibitors, agents that regulate the degradation of messenger RNA and protease inhibitors are discussed.
Similar content being viewed by others
References
Van der Lubbe PA, Dijkmans BAC, Markusse HM, et al. A randomized, double-blind, placebo-controlled study of CD4 monoclonal antibody therapy in early rheumatoid arthritis. Arthritis Rheum 1995; 38: 1097–106
Chu CQ, Field M, Allard S, et al. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol 1992; 31: 653–61
Chen E, Keystone EC, Fish EN. Restricted cytokine expression in rheumatoid arthritis. Arthritis Rheum 1993; 36: 901–9
Brennan FM, Chantry D, Jackson A, et al. Inhibitory effect of TNF alpha antibodies on synovial cell IL 1 production in rheumatoid arthritis. Lancet 1989; II: 244–7
Haworth C, Brennan FM, Chantry D, et al. Expression of granulocyte-macrophage colony stimulating factor (GM-CSF) in rheumatoid arthritis: regulation by tumor necrosis factor alpha. Eur J Immunol 1991; 21: 2575–9
Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumor necrosis factor: a predictive model of arthritis. EMBO J 1991; 10: 4025–31
Fong KY, Boey ML, Koh WH, et al. Cytokine concentrations in the synovial fluid and plasma of RA patients: correlation with bony erosions. Clin Exp Rheumatol 1994; 12: 55–8
Bathon JM, Hwang JJ, Shin LH, et al. Type IV collagen-specific mRNA is expressed by cultured synovial fibroblasts and is suppressed by IL-1. Arthritis Rheum 1994; 37: 1350–6
Arner EC, Tortorella MD. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with IL2. Arthritis Rheum 1995; 38: 1304–14
Wendling D, Racadot E, Wijdenes J. Treatment of severe rheumatoid arthritis by anti-IL6 mAb. J Rheumatol 1993; 20: 259–62
Dinarello CA. Modalities for reducing IL1 activity in disease. Immunol Today 1993; 14: 260–4
Bandara G, Mueller GM, Galea-Lauri J, et al. Intraarticular expression of biologically active IL1-RA protein by ex vivo gene transfer. Proc Natl Acad Sci USA 1993; 90: 10764–8
Lebsack ME, Paul CC, Martindale JJ, et al. A dose and regimen ranging study of IL 1-RA in patients with RA. Arthritis Rheum 1993; 36: S39
Drevlow B, Capezio J, Lovis R, et al. Phase I study of recombinant human IL1 receptor administered intraarticularly in active RA. Arthritis Rheum 1993; 36 Suppl.: S39
Drevlow B, Lovis R, Haag MA. Phase I study of recombinant human IL1 receptor administered subcutaneously in patients with active rheumatoid arthritis. Arthritis Rheum 1994; 37: S339
Elliott MJ, Maini RN, Feldmann M, et al. Randomised doubleblind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 1994; 344: 1105–10
Elliott MJ, Maini RN. Anti-cytokines and cytokines in rheumatoid arthritis. Rheumatol Europe 1995; 24: 94–8
Garcia I, Miyazaki Y, Araki K, et al. Transgenic mice expressing high levels of s TNFR1 fusion protein are protected from lethal septic shock and cerebral malaria and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol 1995; 25: 2401–2407
Moreland LW, Margolies GR, Heck IW, et al. Soluble tumor necrosis factor receptor (STNFR): results of a phase I dose escalation study in patients with rheumatoid arthritis. Arthritis Rheum 1994; 37: S295
Rosenstein ED, Kunicka J, Kramer N, et al. Modification of cytokine production by piroxicam. J Rheumatol 1994; 21: 901–4
Marlich GD, Danner RL, Ceska M, et al. Detection of IL8 and TNF in normal humans after intravenous endotoxin: the effect of anti-inflammatory agents. J Exp Med 1991; 173: 1021–4
Spinas GA, Bloesch D, Keller U, et al. Pretreatment with ibuprofen augments circulating TNF, IL6 and elastase during acute endotoxinemia. J Infect Dis 1991; 163: 89–95
Madhok R. Tenidap. Lancet 1995; 346: 481–5
Zhu X, Ertel W, Ayala A, et al. Chloroquine inhibits macrophage TNFα mRNA transcription. Immunology 1993; 80: 122–6
Clark P, Casas E, Tugwell P, et al. Hydroxychloroquine compared with placebo in rheumatoid arthritis: a randomized controlled trial. Ann Intern Med 1993; 119: 1067–77
Neuman PM, To SST, Robinson GM, et al. Effect of gold sodium thiomalate and its thiomalate component on the in vitro expression of endothelial cell adhesion molecules. J Clin Invest 1994; 94: 1864–7
Handel ML, Watts CKW, Defazio A, et al. Inhibition of AP-1 binding and transcription by gold and selenium involving conserved cysteine residues in Jun and Fose. Proc Natl Acad Sci USA 1995; 92: 4497–501
Yanni G, Farahat MNMR, Poston RN, et al. Intramuscular gold decreases cytokine expression and macrophage numbers in the rheumatoid synovial membrane. Ann Rheum Dis 1994; 53: 256–60
Capell HA, Porter DR, Madhok R, et al. Second line (diseasemodifying) treatment in rheumatoid arthritis: which drug for which patient. Ann Rheum Dis 1993; 52: 423–9
Rains CP, Noble S, Faulds D. Sulfasalazine: a review of its pharmacological properties and therapeutic efficacy in the treatment of rheumatoid arthritis. Drugs 1995; 50: 137–56
Neumann VC, Taggart AJ, Le Gallez P, et al. A study to determine the active moiety of sulphasalazine in rheumatoid arthritis. J Rheumatol 1986; 13: 285–7
Kremer JM. The mechanism of action of methotrexate in rheumatoid arthritis: the search continues. J Rheumatol 1994; 21: 1–5
Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. J Clin Invest 1993; 92: 2675–82
Baggott JE, Vaughn WH, Hudson BB. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J 1986; 236: 193–200
Cronstein BN, Naime D, Firestein G. The antiinflammatory effects of an adenosine kinase inhibitor are mediated by adenosine. Arthritis Rheum 1995; 38: 1040–5
Firestein GS, Pain MM, Boyle DL. Mechanisms of methotrexate action in rheumatoid arthritis: selective decrease in synovial collagenase gene expression. Arthritis Rheum 1994; 37: 193–200
Balsa A, Gamallo C, Martin-Mola E, et al. Histologic changes in rheumatoid synovitis induced by naproxen and methotrexate. J Rheumatol 1993; 30: 1472–77
Ohya N, Yamada T, Takashi K, et al. Differential effects of MTX on the proliferation and proinflammatory cytokine production of an in vitro model for human proliferative synovitis [abstract]. Arthritis Rheum 1995; 38: S265
Barrera P, Boerbooms AM, Demacker PN, et al. Circulating concentrations and production of cytokines and soluble receptors in RA patients: effect of a single dose of MTX. Br J Rheumatol 1994; 33: 1017–24
Barrera P, Boerbooms ATH, Janssen EM, et al. Circulating soluble tumor necrosis factor receptors, IL2 receptors, TNF alpha and IL6 levels in rheumatoid arthritis. Arthritis Rheum 1993; 36: 1070–9
Weinblatt ME, Kaplan H, Germain BF, et al. Methotrexate in rheumatoid arthritis: a five-year prospective multicenter study. Arthritis Rheum 1994; 37: 1492–8
Cabie A, Farkas JC, Fitting C, et al. High levels of portal TNF-alpha during abdominal aortic surgery in man. Cytokine 1993; 5: 448–53
Landewé RBM, Goei The HS, van Rijthoven AM, et al. A randomized, double-blind, 24-week controlled study of low dose cyclosporine versus chloroquine for early rheumatoid arthritis. Arthritis Rheum 1994; 37: 637–43
Zuckerman SH, Shellhaas J, Butler LD. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticosteroids and the role of the pituitary-adrenal axis. Eur J Immunol 1989; 19: 301–5
Colding S, Emery R, Young SP. Tenidap-modulated proinflammatory cytokine activation of a monocyte cell line. J Immunol 1995; 154: 5384–90
Miltenburg AMM, Dolhain RJEM, de Kuiper R, et al. Tenidap inhibits T-cell proliferation, cytokine production and the induction of mRNA encoding TNF and IFN-gamma [abstract]. Arthritis Rheum 1994; 37: S384
Laliberte R, Perrigan XD, Svensson L, et al. Tenidap modulates cytoplasmic pH and inhibits anion transport in vitro. 2. Inhibition of IL1 production from ATP-treated monocytes and macrophages. J Immunol 1994; 153: 2168–79
Conti P, Reale M, Barbacane RC, et al. Effect of tenidap on CD3, CD4 and CD8 expression and IL1 and LB4 secretion in PBMC. Biochem Cell Biol 1994; 72: 397–402
Sack U, Kuhn H, Ermann J, et al. Synovial tissue implants from patients with rheumatoid arthritis cause cartilage destruction in knee joints of SCID.bg mice. J Rheumatol 1994; 21: 10–6
Bouma MG, Stad RK, van den Wildenberg FAJM, et al. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol 1994; 153: 4159–68
Prabhakar U, Brooks DP, Lipshlitz D, et al. Inhibition of LPS-induced TNF alpha production in human monocytes by adenosine (A(2)) receptor selective agonists. Int J Immunopharmacol 1995; 17: 221–4
Rosengren S, Bong GW, Firestein GS. Anti-inflammatory effects of an adenosine kinase inhibitor: decreased neutrophil accumulation and vascular leakage. J Immunol 1995; 154: 5444–51
Thiel M. Acting via A2 receptor, adenosine inhibits the production of TNFα of endotoxin stimulated PMN. J Lab Clin Med 1995; 126: 275–82
Sommer N, Loschmann PA, Northoff GH, et al. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nature Med 1995; 1: 244–8
Landman D, Sarai A, Sathe SS. Use of pentoxifylline therapy for patients with AIDS-related wasting: pilot study. Clin Infect Dis 1994; 18: 97–9
Maksymowych WP, Avinazubieta A, Luong MH, et al. An open study of pentoxifylline in the treatment of severe refractory rheumatoid arthritis. J Rheumatol 1995; 22: 625–9
Eugui EM, Delustro B, Rouhafza S, et al. Coordinate inhibition by some antioxidants of TNF alpha, IL-1 beta, and IL-6 production by human peripheral blood mononuclear cells. Immunosuppressive Antiinflammatory Drugs 1993; 696: 181–4
Eugui EM, Delustro B, Rouhafza S, et al. Some antioxidants inhibit, in a co-ordinate fashion, the production of tumor necrosis factor-alpha, IL-beta, and IL-6 by human peripheral blood mononuclear cells. Int Immunol 1994; 6: 409–22
Zhang HB, Spapen H, Nguyen DN, et al. Protective effects of N-acetyl-L-cysteine in endotoxemia. Am J Physiol 1994; 266: H1746–54
Marchant A, Bruyns C, Vandenabeele P, et al. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol 1994; 24: 1167–71
School LB, Albrecht H, Gallay P, et al. Cytokine regulation of TNF-alpha mRNA and protein production by unprimed macrophages from C57BI/6 and NZW mice. J Leukocyte Biol 1994; 56: 514–20
Chernoff AE, Granowitz EV, Shapiro L, et al. A randomized, controlled trial of IL-10 in humans: inhibition of inflammatory cytokine production and immune responses. J Immunol 1995; 154: 5492–9
Moreira AL, Sampaio EP, Zmuidzinas A, et al.Thalidomide exerts an inhibitory action on TNFα by enhancing mRNA degradation. J Exp Med 1993: 177; 1675–80
Revuz J, Guillaume J, Janier M, et al. Crossover study of thalidomide vs placebo in severe recurrent aphthous stomatitis. Arch Dermatol 1990: 126; 923–7
Guttiérrez-Rodriguez O, Starusla-Bacal P, Guttiαrrez-Monter O. Treatment of refractory RA: the thalidomide experience. J Rheumatol 1989; 16: 156–63
Mohler KM, Sleath PR, Fitzner JN, et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 1994; 370: 218–20
Gearing AJH, Beckett P, Christodowlow M, et al. Matrix metalloproteinases and processing of pro-TNF-α. J Leukocyt Biol 1995; 57: 774–7
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huizinga, T.W.J., Breedveld, F.C. Cytokine-Suppressive Anti-Inflammatory Drugs. Clin. Immunother. 6, 395–404 (1996). https://doi.org/10.1007/BF03259358
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03259358