Summary
-
▲ Leflunomide is a novel isoxazole derivative with immunosuppressant and anti-inflammatory properties. It has been most extensively evaluated in patients with rheumatoid arthritis and may be categorised as a disease-modifying antirheumatic drug (DMARD).
-
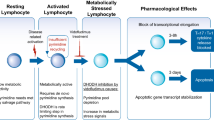
▲ The drug appears to have a unique mechanism of action involving inhibition of de novo pyrimidine synthesis in immune cells.
-
▲ In animal models of arthritis, leflunomide demonstrated a number of beneficial effects including reduced inflammation and joint stiffness.
-
▲ In patients with rheumatoid arthritis, leflunomide 10 or 25 mg/day for 24 weeks significantly improved various outcome measures such as patient global assessment and swollen joint scores compared with placebo. Efficacy seems to be similar to that of established DMARDs.
-
▲ Relative to established DMARDs, leflunomide appears to have a rapid onset of action. The most frequently reported adverse events with leflunomide are gastrointestinal.
Similar content being viewed by others
References
Bartlett RR, Anagnostopulos H, Zielinski T, et al. Effects of leflunomide on immune responses and models of inflammation. Springer Semin Immunopathol 1993 Apr; 14: 381–94
Cao WW, Kao PN, Chao AC, et al. Mechanism of the antiproliferative action of leflunomide: A77 1726, the active metabolite of leflunomide, does not block T-cell receptor-mediated signal transduction but its antiproliferative effects are antagonized by pyrimidine nucleosides. J Heart Lung Transplant 1995 Nov–Dec; 14 (Pt 1): 1016–30
Cherwinski HM, Cohn RG, Cheung P, et al. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J Pharmacol Exp Ther 1995 Nov; 275: 1043–9
Cherwinski HM, Byars N, Ballaron SJ, et al. Leflunomide interferes with pyrimidine mucleotide biosynthesis. Inflamm Res 1995 Aug; 44: 317–22
Morris RE. Mechanisms of action of new immunosuppressive drugs. Kidney Int 1996; 49 Suppl. 53: S26–38
Zielinski T, Zeitter D, Mullner S, et al. Leflunomide, a reversible inhibitor of pyrimidine biosynthesis? Inflamm Res 1995; 44 Suppl. 2: S207–8
Greene S, Watanabe K, Braatztrulson J, et al. Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochem Pharmacol 1995 Sep 7; 50: 861–7
Atluru S, Atluru D. Evidence that genistein, a protein-tyroxine kinase inhibitor, inhibits CD28 monoclonal-antibody-stimulated human T cell proliferation. Transplantation 1991; 51(2): 448–50
Cao W, Chao AC, Morris RE. Leflunomide, a new immunosuppressant, inhibits tyrosine kinase, calcium signaling and DNA synthesis in vascular smooth muscle [abstract]. FASEB J 1994 Mar 15; 8 (Pt 1): 486
Mattar T, Kochhar K, Bartlett R, et al. Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett 1993 Nov 15; 334: 161–4
Bartlett RR, Schleyerbach R. Immunopharmacological profile of a novel isoxazol derivative, HWA 486, with potential antirheumatic activity. I. Disease modifying action on adjuvant arthritis of the rat. Int J Immunopharmacol 1985; 7: 7–18
Pasternak RD, Wadopian NS, Wright RN, et al. Disease modifying activity of HWA 486 in rat adjuvant-induced arthritis. Agents Actions 1987 Aug; 21: 241–3
Giant TT, Mikecz K, Bartlett RR, et al. Immunomodulation of proteoglycan-induced progressive polyarthritis by leflunomide. Immunopharmacology 1992 Mar–Apr; 23: 105–16
Giant TT, Mikecz K, Brennan F, et al. Suppression of autoimmune responses and inflammatory events by leflunomide in an animal model for rheumatoid arthritis. Agents Actions 1994 Aug; 41 (Spec. issue): C267–70
Dimitrijevic M, Kosec D, Popeskovic L, et al. Influence of leflunomide (HWA 486) on immune system activation in RA [abstract]. Rev Esp Reumatol 1993 Jul; 20 Suppl. 1: 398
Dimitrijevic M, Stupar N, Pilipovic N. Leflunomide in the treatment of patients with severe rheumatoid arthritis: influence on immunological parameters [abstract]. Clin Exp Rheumatol 1995 Sep–Oct; 13 Suppl. 12: 56
Richardson C, Emery P. New therapies for rheumatoid arthritis. Br J Clin Pract 1995; 49(3): 135–9
Furst DE. Cyclosporin, leflunomide and nitrogen mustard. Baillieres Clin Rheumatol 1995 Nov; 9: 711–29
Mladenovic V, Domljan Z, Rozman B, et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis: results of a randomized, placebo-controlled, phase II study. Arthritis Rheum 1995 Nov; 38: 1595–603
Carlson RP. Newer immunosuppressive drugs and other agents for the treatment of rheumatoid arthritis — an update. Expert Opin Invest Drug 1995 Sep; 4: 853–9
Lucien J, Dias VC, LeGatt DF. Blood distribution and single-dose pharmacokinetics of leflunomide. Ther Drug Monit 1995 Oct; 17: 454–9
Roethig H-J, Collins J, Harnisch L, et al. The effect of activated charcoal and cholestyramine on the pharmacokinetics of leflunomide [abstract no. PIII-65]. Clin Pharmacol Ther 1996; 59(2): 204
Campion G, Löw-Friedrich I, Oed C, et al. Comparison of different dosing regimens of leflunomide in the treatment of active rheumatoid arthritis [abstract]. Scand J Rheumatol 1994 Suppl. 98: abstr. 140
Domljan Z, Popovic M, Mladenovic V, et al. Efficacy and safety of leflunomide in the treatment of patients with rheumatoid arthritis (RA) [abstract]. Arthritis Rheum 1993 Sep; 36 Suppl.: 56
Dias VC, Lucien J, LeGatt DF, et al. Measurement of the active leflunomide metabolite (A77 1726) by reverse-phase high-performance liquid chromatography. Ther Drug Monit 1995 Feb; 17: 84–8
Dekleva-Djordjevic J, Radunovic L, Musikic P, et al. Adverse effects of leflunomide treatment in patients with rheumatoid arthritis at the Zemun clinical centre [abstract]. Clin Exp Rheumatol 1995 Sep–Oct; 13 Suppl. 12: 57
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Plosker, G.L., Wagstaff, A.J. Leflunomide. Clin. Immunother. 6, 300–305 (1996). https://doi.org/10.1007/BF03259090
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03259090