Summary
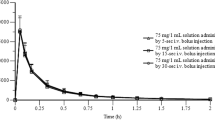
Bioavailability and pharmacokinetic studies with piroxicam-β-cyclodextrin (CHF 1194) showed that absorption of piroxicam administered as a complex is faster than that of piroxicam alone for the oral and rectal routes of administration in rabbits and dogs. This inclusion complex, which has a molar ratio of 1:2.5, produced the optimal absorption of all the combinations tested. CHF 1194 has excellent bioavailability by the 2 routes tested and the kinetics are characterised by a rapid onset of peak plasma concentrations that are greater than those obtained with piroxicam alone.
Similar content being viewed by others
References
Dansions AC, Johnson EL, Keeley FJ, Gryczko Rawsky V. High performance liquid chromatographic analysis of isoxicam in human plasma and urine. Journal of Chromatography 305: 145, 1984
Duchêne D, Glomot F, Vaution C. Pharmaceutical applications of cyclodextrins. In Duchêne D (Ed.) Cyclodextrins and their industrial uses, pp. 211–257, Editions de Santé, Paris, 1987
Hobbs DC, Twomey TM. Piroxicam pharmacokinetics in man: aspirin and antacid interaction studies. Journal of Clinical Pharmacology 19: 270–281, 1979
Hobbs DC, Twomey TM. Metabolism of piroxicam by laboratory animals. Drug Metabolism and Disposition 9: 114–118, 1981
Koelle EU, Hengy H, Vollmer KO. Pharmacokinetics of isoxicam in man following oral administration. Arzneimittel-Forschung 33: 528, 1983
Pitha J, Szente L, Szejtli J. Molecular encapsulation of drugs by cyclodextrins and congeners. In Bruck SD (Ed.) Controlled drug delivery, Vol. 1, pp. 125–148, CRC Press Inc., Boca Raton, Florida, 1983
Richardson CJ, Sharon GR, Blocka KL, Verbeeck RK. High-performance liquid chromatographic analysis of piroxicam and its major metabolite 5′-hydroxypiroxicam in human plasma and urine. Journal of Chromatography 382: 383–385, 1986
Schiantarelli P, Acerbi D, Bovis G. Some pharmacokinetic properties and bioavailability by oral and rectal route of piroxicam in rodents and in man. Arzneimittel-Forschung 31: 92, 1981
Szejtli J. Cyclodextrins and their inclusion complexes. Akademiai Kiado, Budapest, 1982
Szejtli J. Industrial applications of cyclodextrins. In Atwood JL et al. (Eds) Inclusion compounds, Vol. 3, pp. 331–390, Academic Press, London, 1984
Uekama K, Otagiri M. Cyclodextrins in drug carrier systems. CRC Critical Reviews in Therapeutic Drug Carrier Systems 3: 1–40, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Acerbi, D. Pharmacokinetic Profile of Piroxicam-β-Cyclodextrin. Drug Invest 2 (Suppl 4), 42–49 (1990). https://doi.org/10.1007/BF03258226
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03258226