Abstract
Background and Objective: Chemotherapy for advanced non-small-cell lung cancer (NSCLC) remains marginally effective, with a 5-year overall survival rate of approximately 5%. Recently, the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib was approved in Slovakia for the treatment of metastatic NSCLC. Gefitinib is a selective EGFR inhibitor that binds to the adenosine triphosphate binding pocket of the kinase domain and blocks downstream signaling pathways. Mutations of the EGFR gene, particularly an in-frame 15 bp deletion (delE746_A750) in exon 19 and the L858R mutation in exon 21, correlate with enhanced clinical responsiveness to EGFR tyrosine kinase inhibitors. However, the detection of these mutations and thereby prediction of the therapy outcome is sometimes unreliable due to the low sensitivity of direct sequencing if the proportion of tumor cells in the tissue is less than 25%. Therefore we decided to test the applicability of other methods, particularly high-resolution melting analysis (HRMA), for detection of these mutations in clinical samples.
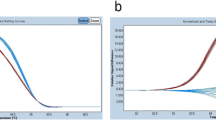
Methods: We analyzed 53 archival cytologic specimens for the presence of EGFR mutations, using the HRMA method. Results were verified by direct sequencing. For samples containing less than 25% tumor cells, we used mutant-enriched PCR before sequencing. We also performed a titration assay to establish the lower limit of the proportion of tumor cells for detection of EGFR mutations.
Results: EGFR mutations were detected in 13 cases (24%). In-frame deletions in exon 19 were detected in eight cases (15%) and the L858R mutation in exon 21 was detected in five cases (9%). The positive results of the HRMA were confirmed by direct sequencing only in five of 13 cases. In the remaining eight positive samples, HRMA results were confirmed by sequencing analysis after mutant-DNA enrichment. The titration assay established that the lower limit for detection of EGFR mutations by HMRA was 1% tumor cells in the clinical sample.
Conclusion: Our results indicated that HRMA in combination with mutant-enriched PCR represents a sensitive method for detection of EGFR mutations from cytologic specimens. When properly executed, this protocol allows identification of EGFR mutations in specimens containing a minimal percentage of tumor cells.





Similar content being viewed by others
References
National Health Information Center. Health statistics yearbook of the Slovak Republic 2008. Bratislava: National Health Information Center, 2009: 208
Uddin D. Non-small cell lung cancer (NSCLC): an update. TAJ: Journal of Teachers Association 2002; 15: 115-8 [online]. Available from URL: http://www.banglajol.info/index.php/TAJ/article/view/3923 [Accessed 2011 Mar 1]
Jemal A, Siegel R, Ward E. Cancer statistics. CA Cancer J Clin 2007; 57:43–66
Uramoto H, Mitsudomi T. Which biomarker predicts benefit from EGFR-TKI treatment for patients with lung cancer? Br J Cancer 2007; 96: 857–63
Katzel JA, Fanutcchi MP, Li Z. Recent advances of novel targeted therapy in non-small cell lung cancer. J Hematol Oncol 2009; 2: 1–18
Gazdar AF, Minna JD. Deregulated EGFR signaling during lung cancer progression: mutations, amplicons and autocrine loops. Cancer Prev Res 2008; 1: 156–60
Nair P. Epidermal growth factor receptor family and its role in cancer progression. Current Science 2005; 88: 890–8
Neal JW, Sequist V. Targeted therapies: optimal first-line therapy for NSCLC with EGFR mutations. Nat Rev Clin Oncol 2010; 7: 71–2
Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation and clinical response to gefitinib therapy. Science 2004; 304: 1497–500
Yun CH, Boggon TJ, Li Y, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 2007; 11: 217–27
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor underlying responsiveness of NSCLC to gefitinib. N Engl J Med 2004; 350: 2129–39
Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004; 101: 13306–11
Reed GH, Kent JO, Wittwer CT. High resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics 2007; 8: 597–608
Wittwer CT, Reed GH, Gundry CN, et al. High resolution genotyping by amplicon melting analysis using LC Green. Clin Chem 2003; 49: 853–60
Kimura H, Fijiwara Y, Sone T, et al. High sensitivity detection of EGFR mutations in the pleural effusion of NSCLC patients. Cancer Sci 2006; 97: 642–8
Cohen V, Aqulnik JS, Jarry J, et al. Evaluation of denaturating high-performance liquid chromatography as a rapid detection method for identification of EGFR mutations in NSCLC. Cancer 2006; 107: 2858–65
Asano H, Toyooka S, Tokumo M, et al. Detection of EGFR gene mutation in lung cancer by mutant-enriched PCR polymerase chain reaction assay. Clin Cancer Res 2006; 12: 43–8
Nomoto K, Tsuta K, Takano T, et al. Detection of EGFR mutations in archieved cytologic specimens of NSCLC using HRMA. Am J Clin Pathol 2006; 126: 608–15
Fukui T, Ohe Y, Tsuta K, et al. Prospective study of the accuracy of EGFR mutational analysis by high-resolution melting analysis in small samples obtained from patients with non-small cell lung cancer. Clin Cancer Res 2008; 14:4751–7
Heideman DAM, Thunnisen FB, Doeleman M, et al. A panel of high resolution melting (HRM) technology-based assays with direct sequencing possibility for effective mutation screening of EGFR and K-ras genes. Cellular Oncology 2009; 31: 329–33
Fassina A, Gazziero A, Zardo D, et al. Detection of EGFR and KRAS mutations on trans-thoracic needle aspiration of lung nodules by high resolution melting analysis. J Clin Pathol 2009; 62: 1096–102
Takano T, Ohe Y, Fukui T, et al. Epidermal growth factor receptor mutation detection using high-resolution melting analysis predicts outcomes in patients with advanced non-small cell lung cancer treated with gefitinib. Clin Cancer Res 2007; 13: 5385–90
Chin TM, Anuar D, Soo R, et al. Detection of epidermal growth factor receptor variations by partially denaturing HPLC. Clin Chem 2007; 53: 62–70
Janne PA, Borras AM, Kuang Y, et al. A rapid and sensitive enzymatic method for epidermal growth factor receptor mutation screening. Clin Cancer Res 2006; 12: 751–8
Takano T, Ohe T, Sakamoto H, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol 2005; 23: 6829–37
Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005; 23: 857–65
Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn 2005; 7: 396–403
Miyazawa H, Tanaka T, Nagai Y, et al. Peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based detection test for gefitinib-refractory T790M epidermal growth factor receptor mutation. Cancer Science 2008; 99: 595–600
Endo K, Konishi A, Sasaki H, et al. Epidermal growth factor receptor gene mutation in non-small cell lung cancer using highly sensitive and fast TaqMan PCR assay. Lung Cancer 2005; 50: 375–84
Kimura H, Kasahara K, Kawaishi M, et al. Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res 2006; 12: 3915–21
Do H, Krypuy M, Mitchell PL, et al. High resolution melting analysis for rapid and sensitive EGFR and KRAS mutation detection in formalin fixed paraffin embedded biopsies. BMC Cancer 2008; 8: 1–14
Do H, Dobrovic A. Limited copy number-high resolution melting (LCN-HRM) enables the detection and identification by sequencing of low level mutations in cancer biopsies. Mol Cancer 2009; 8: 1–11
Quach N, Goodman F, Shibata D. In vitro mutation artifacts after formalin fixation and error prone translesion synthesis during PCR. BMC Clin Pathol 2004; 4: 1
Acknowledgments
We thank Jana Tinakova for her help with figure design and Jan Marcus for review and approval of the manuscript. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hlinkova, K., Babál, P., Berzinec, P. et al. Rapid and Efficient Detection of EGFR Mutations in Problematic Cytologic Specimens by High-Resolution Melting Analysis. Mol Diagn Ther 15, 21–29 (2011). https://doi.org/10.1007/BF03257190
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03257190