Abstract
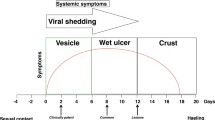
Herpes simplex virus (HSV) is one of the most common, yet frequently overlooked, sexually transmitted infections. Since the type of HSV infection affects prognosis and subsequent counseling, type-specific testing to distinguish HSV-1 from HSV-2 is recommended. Although PCR has been the diagnostic standard for HSV infections of the central nervous system, until now viral culture has been the test of choice for HSV genital infection. However, HSV PCR, with its consistently and substantially higher rate of HSV detection, will likely replace viral culture as the gold standard for the diagnosis of genital herpes in people with active mucocutaneous lesions, regardless of anatomic location or viral type. Alternatively, type-specific serologic tests based on glycoprotein G should be the test of choice to establish the diagnosis of HSV infection when no active lesion is present. Given the difficulty in making the clinical diagnosis of HSV, the growing worldwide prevalence of genital herpes and the availability of effective antiviral therapy, there is an increased demand for rapid, accurate laboratory diagnosis of patients with HSV.



Similar content being viewed by others
References
Center for Disease Control and Prevention. Genital herpes: CDC fact sheet[online]. Available from URL: http://www.cdc.gov/std/Herpes/STDFact-Herpes.htm [Accessed 2005 Oct 10]
Fleming D, McQuillan G, Johnson R, et al. Herpes simplex virus type 2 in theUnited States, 1976 to 1994. N Engl J Med 1997; 337: 1105–11
Ahmed H, Mbwana J, Gunnarsson E, et al. Etiology of genital ulcer disease andassociation with human immunodeficiency virus infection in two Tanzaniancities. Sex Transm Dis 2003; 30(2): 114–9
Beyrer C, Jitwatcharanan K, Natpratan C, et al. Molecular methods for thediagnosis of genital ulcer disease in a sexually transmitted disease clinicpopulation in northern Thailand: predominance of herpes simplex virus infection.J Infect Dis 1998; 178(1): 243–6
Chen C, Ballard R, Beck-Sague C, et al. Human immunodeficiency virus infectionand genital ulcer disease in South Africa: the herpetic connection. Sex TransmDis 2000; 27: 21–9
Corey L. Herpes simplex type 2 infection in the developing world: is it time toaddress this disease? Sex Transm Dis 2000 Jan; 27(1): 30–1
Corey L, Handsfield HH. Genital herpes and public health: addressing a globalproblem. JAMA 2000 Feb 9; 283(6): 791–4
Risbud A, Chan-Tack K, Gadkari D, et al. The etiology of genital ulcer disease bymultiplex polymerase chain reaction and relationship to HIV infection amongpatients attending sexually transmitted disease clinics in Pune, India. SexTransm Dis 1999 Jan; 26(1): 55–62
Langenberg AG, Corey L, Ashley RL, et al. A prospective study of new infectionswith herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group.N Engl J Med 1999 Nov 4; 341(19): 1432–8
Wald A, Zeh J, Selke S, et al. Virologic characteristics of subclinical and symptomaticgenital herpes infections. N Engl J Med 1995 Sep 21; 333(12): 770–5
Brown Z, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection inrelation to asymptomatic maternal infection at the time of labor. N Engl J Med1991; 324: 1247–52
Mertz G, Schmidt O, Jourden J, et al. Frequency of acquisition of first-episodegenital infection with herpes simplex virus from symptomatic and asymptomaticsource contacts. Sex Transm Dis 1985; 12: 33–9
Mullan HM, Munday PE. The acceptability of the introduction of a type specificherpes antibody screening test into a genitourinary medicine clinic in the UnitedKingdom. Sex Transm Infect 2003 Apr; 79(2): 129–33
Cowan F, Johnson A, Ashley R, et al. Antibody to herpes simplex virus type 2 asserological marker of sexual lifestyle in populations. BMJ 1994 Nov 19; 309(6965): 1325–9
Strick L, Wald A. Type-specific testing for herpes simplex virus. Expert Rev MolDiagn 2004; 4(4): 443–53
Lafferty W. The changing epidemiology of HSV-1 and HSV-2 and implications forserological testing. Herpes 2002; 9(2): 51–5
Lafferty W, Downey L, Celum C, et al. Herpes simplex virus type 1 as a cause ofgenital herpes: impact on surveillance and prevention. J Infect Dis 2000; 181:1454–7
Ribes J, Steele A, Seabolt J, et al. Six-year study of the incidence of herpes ingenital and nongenital cultures in a central Kentucky medical center patientpopulation. J Clin Microbiol 2001; 39(9): 3321–5
Ross J, Smith J, Elton R. The epidemiology of herpes simplex types 1 and 2infection of the genital tract in Edinburgh 1978–1991. Genitourin Med 1993;69: 381–3
Scoular A, Leask B, Carrington D. Changing trends in genital herpes due to herpessimplex virus type 1 in Glasgow, 1985–88. Genitourin Med 1990; 66: 226
Vyse A, Gay N, Slomka M, et al. The burden of infection with HSV-1 and HSV-2in England and Wales: implications for the changing epidemiology of genitalherpes. Sex Transm Infect 2000; 76: 183–7
Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomaticfirst-episode infection. Ann Intern Med 1994 Dec 1; 121(11): 847–54
Koutsky L, Stevens C, Holmes K, et al. Underdiagnosis of genital herpes by currentclinical and viral-isolation procedures. N Engl J Med 1992; 326: 1533–9
Sucato G, Wald A, Wakabayashi E, et al. Evidence of latency and reactivation ofboth herpes simplex virus (HSV-1) and HSV-2 in the genital region. J Infect Dis1998; 177: 1069–72
Sexually transmitted diseases treatment guidelines 2002. Center for Disease Controland Prevention. MMWR Recomm Rep 2002 May 10; 51(RR-6): 1–78
Chibo D, Julian D, Sasadeusz J, et al. Molecular analysis of clinical isolates ofacyclovir resistance herpes simplex virus. Antiviral Res 2004; 61: 83–91
Kriesel JD, Spruance SL, Prichard M, et al. Recurrent antiviral-resistant genitalherpes in an immunocompetent patient. J Infect Dis 2005 Jul 1; 192: 156–61
Sasadeusz JJ, Tufaro R, Safrin S, et al. Homopolymer mutational hot spots mediateherpes simplex virus resistance to acyclovir. J Virol 1997 May; 71(5): 3872–8
Wald A, Zeh J, Barnum G, et al. Suppression of subclinical shedding of herpessimplex virus type 2 with acyclovir. Ann Intern Med 1996 Jan 1; 124 (1 Pt 1):8–15
Wald A, Huang ML, Carrell D, et al. Polymerase chain reaction for detection ofherpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSVisolation in cell culture. J Infect Dis 2003 Nov 1; 188(9): 1345–51
Orle KA, Gates CA, Martin DH, et al. Simultaneous PCR detection of Haemophilusducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 fromgenital ulcers. J Clin Microbiol 1996 Jan; 34(1): 49–54
Safrin S, Shaw H, Bolan G, et al. Comparison of virus culture and the polymerasechain reaction for diagnosis of mucocutaneous herpes simplex virus infection.Sex Transm Dis 1997 Mar; 24(3): 176–80
Morse SA, Trees DL, Htun Y, et al. Comparison of clinical diagnosis and standardlaboratory and molecular methods for the diagnosis of genital ulcer disease inLesotho: association with human immunodeficiency virus infection. J InfectDis 1997 Mar; 175(3): 583–9
Slomka MJ, Emery L, Munday PE, et al. A comparison of PCR with virus isolationand direct antigen detection for diagnosis and typing of genital herpes. J MedVirol 1998 Jun; 55(2): 177–83
Waldhuber MG, Denham I, Wadey C, et al. Detection of herpes simplex virus ingenital specimens by type-specific polymerase chain reaction. Int J STD AIDS1999 Feb; 10(2): 89–92
Coyle PV, Desai A, Wyatt D, et al. A comparison of virus isolation, indirectimmunofluorescence and nested multiplex polymerase chain reaction for thediagnosis of primary and recurrent herpes simplex type 1 and type 2 infections.J Virol Methods 1999 Dec; 83(1-2): 75–82
Ryncarz AJ, Goddard J, Wald A, et al. Development of a high-throughput quantitativeassay for detecting herpes simplex virus DNA in clinical samples. J ClinMicrobiol 1999 Jun; 37(6): 1941–7
Markoulatos P, Georgopoulou A, Kotsovassilis C, et al. Detection and typing ofHSV-1, HSV-2, and VZV by a multiplex polymerase chain reaction. J Clin LabAnal 2000; 14(5): 214–9
Espy MJ, Ross TK, Teo R, et al. Evaluation of LightCycler PCR for implementationof laboratory diagnosis of herpes simplex virus infections. J Clin Microbiol2000 Aug; 38(8): 3116–8
Espy MJ, Uhl JR, Mitchell PS, et al. Diagnosis of herpes simplex virus infections inthe clinical laboratory by LightCycler PCR. J Clin Microbiol 2000 Feb; 38(2):795–9
Marshall DS, Linfert DR, Draghi A, et al. Identification of herpes simplex virusgenital infection: comparison of a multiplex PCR assay and traditional viralisolation techniques. Mod Pathol 2001 Mar; 14(3): 152–6
Coyle PV, O’Neill HJ, McCaughey C, et al. Clinical utility of a nested nucleic acidamplification format in comparison to viral culture for the diagnosis of mucosalherpes simples infection in a genitourinary medicine setting. BMC Infect Dis2001; 1(11): 1471–2334
Espy MJ, Rys PN, Wold AD, et al. Detection of herpes simplex virus DNA ingenital and dermal specimens by LightCycler PCR after extraction using theIsoQuick, MagNA Pure, and BioRobot 9604 methods. J Clin Microbiol 2001Jun; 39(6): 2233–6
Nicoll S, Brass A, Cubie HA. Detection of herpes viruses in clinical samples usingreal-time PCR. J Virol Methods 2001; 96: 25–31
Druce J, Catton M, Chibo D, et al. Utility of a multiplex PCR assay for detectingherpesvirus DNA in clinical samples. J Clin Microbiol 2002 May; 40(5):1728–32
Scoular A, Gillespie G, Carman WF. Polymerase chain reaction for diagnosis ofgenital herpes in a genitourinary medicine clinic. Sex Transm Infect 2002 Feb;78(1): 21–5
Burrows J, Nitsche A, Bayly B, et al. Detection and subtyping of Herpes simplexvirus in clinical samples by LightCycler PCR, enzyme immunoassay and cellculture. BMC Microbiol 2002 Jun 9; 2(1): 12
van Doornum GJ, Guldemeester J, Osterhaus AD, et al. Diagnosing herpesvirusinfections by real-time amplification and rapid culture. J Clin Microbiol 2003Feb; 41(2): 576–80
Mengelle C, Sandres-Saune K, Miedouge M, et al. Use of two real-time polymerasechain reactions (PCRs) to detect herpes simplex type 1 and 2-DNA afterautomated extraction of nucleic acid. J Med Virol 2004 Nov; 74(3): 459–62
Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by realtime PCR in routine clinical practice. Sex Transm Infect 2004 Oct; 80(5):406–10
Kimberlin DW, Lakeman FD, Arvin AM, et al. Application of the polymerasechain reaction to the diagnosis and management of neonatal herpes simplexvirus disease. National Institute of Allergy and Infectious Diseases CollaborativeAntiviral Study Group. J Infect Dis 1996 Dec; 174(6): 1162–7
Mitchell PS, Espy MJ, Smith TF, et al. Laboratory diagnosis of central nervoussystem infections with herpes simplex virus by PCR performed with cerebrospinalfluid specimens. J Clin Microbiol 1997 Nov; 35(11): 2873–7
Whitley RJ, Lakeman F. Herpes simplex virus infections of the central nervoussystem: therapeutic and diagnostic considerations. Clin Infect Dis 1995 Feb; 20(2): 414–20
Cinque P, Cleator GM, Weber T, et al. The role of laboratory investigation in thediagnosis and management of patients with suspected herpes simplex encephalitis:a consensus report. The EU Concerted Action on Virus Meningitis andEncephalitis. J Neurol Neurosurg Psychiatry 1996 Oct; 61(4): 339–45
Whitley RJ. Viral encephalitis. N Engl J Med 1990 Jul 26; 323(4): 242–50
Revello MG, Baldanti F, Sarasini A, et al. Quantitation of herpes simplex virusDNA in cerebrospinal fluid of patients with herpes simplex encephalitis by thepolymerase chain reaction. Clin Diagn Virol 1997 Feb; 7(3): 183–91
Aslanzadeh J, Osmon DR, Wilhelm MP, et al. A prospective study of the polymerasechain reaction for detection of herpes simplex virus in cerebrospinal fluidsubmitted to the clinical virology laboratory. Mol Cell Probes 1992 Oct; 6(5):367–73
Rose JW, Stroop WG, Matsuo F, et al. Atypical herpes simplex encephalitis:clinical, virologic, and neuropathologic evaluation. Neurology 1992 Sep; 42(9): 1809–12
Rowley AH, Whitley RJ, Lakeman FD, et al. Rapid detection of herpes simplexvirus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis.Lancet 1990; 335: 440–1
Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application ofpolymerase chain reaction to cerebrospinal fluid from brain-biopsied patientsand correlation with disease. National Institute of Allergy and InfectiousDiseases Collaborative Antiviral Study Group. J Infect Dis 1995; 171: 857–63
Puchhammer-Stöckl E, Presteri E, Croy C, et al. Screening for possible failure ofherpes simplex virus PCR in cerebrospinal fluid for the diagnosis of herpessimplex encephalitis. J Med Virol 2001; 64(4): 531–6
Cone RW, Hobson AC, Palmer J, et al. Extended duration of herpes simplex virusDNA in genital lesions detected by the polymerase chain reaction. J Infect Dis1991 Oct; 164(4): 757–60
Jerome KR, Huang ML, Wald A, et al. Quantitative stability of DNA afterextended storage of clinical specimens as determined by real-time PCR. J ClinMicrobiol 2002 Jul; 40(7): 2609–11
Scoular A. Using the evidence base on genital herpes: optimising the use ofdiagnostic tests and information provision. Sex Transm Infect 2002 Jun; 78(3):160–5
Wald A, Corey L, Cone R, et al. Frequent genital herpes simplex virus 2 sheddingin immunocompetent women: effect of acyclovir treatment. J Clin Invest 1997Mar1; 99(5): 1092–7
Cone RW, Hobson AC, Brown Z, et al. Frequent detection of genital herpessimplex virus DNA by polymerase chain reaction among pregnant women.JAMA 1994 Sep 14; 272(10): 792–6
Kessler HH, Pierer K, Weber B, et al. Detection of herpes simplex virus DNA fromcerebrospinal fluid by PCR and a rapid, nonradioactive hybridization technique.J Clin Microbiol 1994 Aug; 32(8): 1881–6
Sakrauski A, Weber B, Kessler HH, et al. Comparison of two hybridization assaysfor the rapid detection of PCR amplified HSV genome sequences from cerebrospinalfluid. J Virol Methods 1994 Dec; 50(1-3): 175–84
Higuchi R, Dollinger G, Walsh PS, et al. Simultaneous amplification and detectionof specific DNA sequences. Nat Biotech 1992 Apr; 10(4): 413–7
O’Neill HJ, Wyatt DE, Coyle PV, et al. Real-time nested multiplex PCR for thedetection of herpes simplex virus types 1 and 2 and varicella zoster virus. J MedVirol 2003 Dec; 71(4): 557–60
Wittwer CT, Herrmann MG, Moss AA, et al. Continuous fluorescence monitoringof rapid cycle DNA amplification. Biotechniques 1997 Jan; 22(1): 130–1,134-8
Schalasta G, Arents A, Schmid M, et al. Fast and type-specific analysis of herpessimplex virus types 1 and 2 by rapid PCR and fluorescence melting-curve-analysis.Infection 2000 Mar–Apr; 28(2): 85–91
Ririe KM, Rasmussen RP, Wittwer CT. Product differentiation by analysis of DNAmelting curves during the polymerase chain reaction. Anal Biochem 1997 Feb15; 245(2): 154–60
Anderson TP, Werno AM, Beynon KA, et al. Failure to genotype herpes simplexvirus by real-time PCR assay and melting curve analysis due to sequencevariation within probe binding sites. J Clin Microbiol 2003 May; 41(5): 2135–7
Issa NC, Espy MJ, Uhl JR, et al. Sequencing and Resolution of amplified herpessimplex virus DNA with intermediate melting curves as genotype 1 or 2 byLightCycler PCR assay. J Clin Microbiol 2005 Apr; 43(4): 1843–5
Markoulatos P, Siafakas N, Moncany M. Multiplex polymerase chain reaction: apractical approach. J Clin Lab Anal 2002; 16: 47–51
Corey L, Huang ML, Selke S, et al. Differentiation of herpes simplex virus types 1and 2 in clinical samples by a real-time Taqman PCR assay. J Med Virol 2005Jul; 76(3): 350–5
Stocher M, Leb V, Bozic M, et al. Parallel detection of five human herpes virusDNAs by a set of real-time polymerase chain reactions in a single run. J ClinVirol 2003 Jan; 26(1): 85–93
Chou S, Waldemer R, Senters A, et al. Cytomegalovirus UL97 phosphotransferasemutations that affect susceptibility to ganciclovir. J Infect Dis 2002; 185: 162–9
Shafer R. Genotypic testing for human immunodeficiency virus type 1 resistance.Clin Microbiol Rev 2002; 15: 247–77
ACOG practice bulletin. Management of herpes in pregnancy. Number 8 October1999. Clinical management guidelines for obstetrician-gynecologists. Int JGynaecol Obstet 2000 Feb; 68(2): 165–73
Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesareandelivery on transmission rates of herpes simplex virus from mother to infant.JAMA 2003 Jan 8; 289(2): 203–9
Nahmias AJ, Josey W, Naib Z, et al. Perinatal risk associated with maternal genitalherpes simplex virus infection. Am J Obstet Gynecol 1971 Jul 15; 110(6):825–37
Whitley RJ. Neonatal herpes simplex virus infections: pathogenesis and therapy.Pathol Biol (Paris) 1992 Sep; 40(7): 729–34
Stagno S, Whitley R. Herpesvirus infection of pregnancy: part II. Herpes simplexvirus and varicella-zoster infections. N Engl J Med 1985 Nov 21; 313(21):1327–30
Whitley RJ. Neonatal herpes simplex virus infections. Clin Perinatol 1988 Dec; 15(4): 903–16
Nahmias AJ, Whitley RJ, Visintine AN, et al. Herpes simplex virus encephalitis:laboratory evaluations and their diagnostic significance. J Infect Dis 1982 Jun;145(6): 829–36
Malm G, Forsgren M, el Azazi M, et al. A follow-up study of children withneonatal herpes simplex virus infections with particular regard to late nervousdisturbances. Acta Paediatr Scand 1991 Feb; 80(2): 226–34
Corey L, Whitley RJ, Stone EF, et al. Difference between herpes simplex virus type1 and type 2 neonatal encephalitis in neurological outcome. Lancet 1988 Jan2-9; I(8575-6): 1–4
Gardella C, Brown ZA, Wald A, et al. Poor correlation between genital lesions anddetection of herpes simplex virus in women in labor. Am J Obstet Gynecol 2005Aug; 106(2): 268–74
Randolph A, Washington A, Prober C. Cesarean delivery for women presentingwith genital herpes lesions: efficacy, risks, and costs. JAMA 1993; 270: 77–82
Catalano P, Merritt A, Mead P. Incidence of genital herpes simplex virus at thetime of delivery in women with known risk factors. Am J Obstet Gynecol 1991;164 (5 Pt 1): 1301–6
Arvin A, Hensleigh P, Prober C, et al. Failure of antepartum maternal cultures topredict the infant’s risk of exposure to herpes simplex virus at delivery. N Engl JMed 1986 Sep 25; 315(13): 796–800
Hardy DA, Arvin AM, Yasukawa LL, et al. Use of polymerase chain reaction forsuccessful identification of asymptomatic genital infection with herpes simplexvirus in pregnant women at delivery. J Infect Dis 1990 Nov; 162(5): 1031–5
Prober C, Corey L, Brown Z, et al. The management of pregnancies complicated bygenital infections with herpes simplex virus. Clin Infect Dis 1992; 15: 1031–8
Malm G, Forsgren M. Neonatal herpes simplex virus infections: HSV DNA incerebrospinal fluid and serum. Arch Dis Child Fetal Neonatal Ed 1999 Jul; 81(1): F24–9
Diamond C, Mohan K, Hobson A, et al. Viremia in neonatal herpes simplex virusinfections. Pediatr Infect Dis J 1999 Jun; 18(6): 487–9
Bale JF, Miner LJ. Herpes simplex virus infections of the newborn. Curr TreatOptions Neurol 2005 Mar; 7(2): 151–6
Turnback P, Liljeqvest J, Lowhagen G, et al. Glycoprotein G of herpes simplextype 1: identification of type-specific epitopes by human antibodies. J GenVirol 2000; 81: 1033–40
Liljeqvest J, Trybala E, Svennerholm B, et al. Localization of type-specificepitopes of herpes simplex virus type 2 glycoprotein G recognized by humanand mouse antibodies. J Gen Virol 1998; 79: 1215–24
Marsden H, MacAulay K, Murray J, et al. Identification of an immunodominantsequential epitope in glycoprotein G of herpes simplex virus type 2 that is usefulfor serotype-specific diagnosis. J Med Virol 1998; 56: 79–84
Ashley R. Type-specific antibodies to HSV-1 and -2: review of methodology.Herpes 1998; 5: 33–8
Morrow R, Friedrich D. Inaccuracy of certain commercial enzyme immunoassaysin diagnosing genital infections with herpes simplex virus types 1 and 2. Am JClin Pathol 2003; 120(6): 839–44
Ashley R, Cent A, Maggs V, et al. Inability of enzyme immunoassays to discriminatebetween infections with herpes simplex virus type 1 and 2. Ann Intern Med1991; 115: 520–6
Martins T, Woolstenhulme R, Jaskowski T, et al. Comparison of four enzymeimmunoassays with a western blot assay for the determination of type-specificantibodies to herpes simplex virus. Am J Clin Pathol 2001; 115: 272–7
Ashley R. Performance and use of HSV type-specific serology test kits. Herpes2002; 9(2): 38–45
Wald A, Ashley R. Serological testing for herpes simplex virus (HSV)-l andHSV-2 infection. Clin Infect Dis 2002; 35: S173–82
Langenberg A, Benedetti J, Jenkins J, et al. Development of clinically recognizablegenital lesions among women previously identified as having ‘asymptomatic’HSV-2 infection. Ann Intern Med 1989; 110: 882–7
Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2infection in asymptomatic seropositive persons. N Engl J Med 2000; 342:844–50
Morrow RA, Friedrich D, Meier A, et al. Use of “biokit HSV-2 Rapid Assay” toimprove the positive predictive value of Focus HerpeSelect HSV-2 ELISA.BMC Infect Dis 2005; 5: 84
Focus Diagnostics, Inc. HerpeSelect® 2 ELISA IgG (English) package insert[online]. Available from URL: http://www.focustechnologies.com/focus/packageInsert/EL0920G.pdf [Accessed 2006 Jan 18]
Ashley-Morrow R, Krantz E, Wald A. Time course of seroconversion byHerpeSelect ELISA after acquisition of genital herpes simplex virus Type 1(HSV-1) or HSV-2. Sex Transm Dis 2003; 30: 310–4
Cherpes T, Ashley R, Meyn L, et al. Longitudinal reliability of Focus glycoproteinG-based type-specific enzyme immunoassays for detection of herpes simplexvirus types 1 and 2 in women. J Clin Microbiol 2003; 41: 671–4
Munday P, Vuddamalay J, Slomka M, et al. Role of type specific herpes simplexvirus serology in the diagnosis and management of genital herpes. Sex TransmInfect 1998; 74: 175–8
Koutsky L, Ashley R, Holmes K, et al. The frequency of unrecognized type 2herpes simplex virus infection among women: implications for the control ofgenital herpes. Sex Transm Dis 1990; 17: 90–4
Wald A, Corey L. Genital herpes. In: Holmes KK, Sparling KF, Mardh PA, et al.,editors. Sexually transmitted diseases. 3rd ed. New York: McGraw-Hill, 1999:285–312
Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2infection in asymptomatic seropositive persons. N Engl J Med 2000; 342:844–50
Mertz G, Coombs R, Ashley R, et al. Transmission of genital herpes in coupleswith one symptomatic and one asymptomatic partner: a prospective study.J Infect Dis 1988; 157: 1169–77
Wald A, Langenberg AG, Link K, et al. Effect of condoms on reducing thetransmission of herpes simplex virus type 2 from men to women. JAMA 2001;285: 3100–6
Wald A, Langenberg AGM, Krantz E, et al. The relationship between condom useand herpes simplex acquisition. Ann Intern Med 2005; 143: 707–13
Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk oftransmission of genital herpes. N Engl J Med 2004; 350: 11–20
Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus duringpregnancy. N Engl J Med 1997; 337: 509–15
Arvin AM, Whitley RJ. Herpes simplex virus infections. In: [eiRemington JS, Klein JO, editors. Infectious diseases of the fetus and newborn. 5th ed. Philadelphia(PA): WB Saunders Co., 2001: 425–46
Kulhanjian J, Soroush V, Au D, et al. Identification of women at unsuspected riskof primary infection with herpes simplex virus type 2 during pregnancy. N EnglJ Med 1992; 326: 916–20
Whitley R, Nahmias A, Visintine A, et al. Natural history of herpes simplex virusinfection of mother and newborn. Pediatrics 1980; 66: 489–94
Brown Z. HSV-2 specific serology should be offered routinely to antenatalpatients. Rev Med Virol 2000; 10: 141–4
Kinghorn G. Should all pregnant women be offered type-specific serologicalscreening for HSV infection? The argument for. Herpes 2002; 9(2): 46–7
Brocklehurst P, Kinghorn G, Carney O, et al. A randomised placebo-controlledtrial of suppressive aciclovir in late pregnancy in women with recurrent genitalherpes infection. Br J Obstet Gynaecol 1998; 105: 275–80
Scott LL, Hollier LM, Mclntire D, et al. Acyclovir suppression to prevent clinicalrecurrences at delivery after first episode genital herpes in pregnancy: an open-labeltrial. Infect Dis Obstet Gynecol 2001; 9(2): 75–80
Scott LL, Hollier LM, Mclntire D, et al. Acyclovir suppression to prevent recurrentgenital herpes at delivery. Infect Dis Obstet Gynecol 2002; 10(2): 71–7
Scott LL, Sanchez PJ, Jackson GL, et al. Acyclovir suppression to prevent cesareandelivery after first-episode genital herpes. Obstet Gynecol 1996 Jan; 87(1):69–73
Sheffield JS, Hollier LM, Hill JB, et al. Acyclovir prophylaxis to prevent herpessimplex virus recurrence at delivery: a systematic review. Obstet Gynecol 2003Dec; 102(6): 1396–403
Braig S, Luton D, Sibony O, et al. Acyclovir prophylaxis in late pregnancyprevents recurrent genital herpes and viral shedding. Eur J Obstet GynecolReprod Biol 2001 May; 96(1): 55–8
Stray-Pederson B. Acyclovir in late pregnancy to prevent neonatal herpes simplex[letter]. Lancet 1990 Sep 22; 336(8717): 756
Watts D, Brown Z, Money D, et al. A double-blind, randomized, placebo-controlledtrial of acyclovir in late pregnancy for the reduction of herpes simplexvirus shedding and cesarean delivery. Am J Obstet Gynecol 2003 Mar; 188(3):836–43
Hook EW, Cannon RO, Nahmias AJ, et al. Herpes simplex virus infection as a riskfactor for human immunodeficiency virus infection in heterosexuals. J InfectDis 1992 Feb; 165(2): 251–5
Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developingworld. Herpes 2004; 11Suppl. 1: 24A–35A
Gwanzura L, McFarland W, Alexander D, et al. Association between humanimmunodeficiency virus and herpes simplex virus type 2 seropositivity amongmale factory workers in Zimbabwe. J Infect Dis 1998; 177: 481–4
Enzensberger R, Braun W, July C, et al. Prevalence of antibodies to humanherpesviruses and hepatitis B virus in patients at different stages of humanimmunodeficiency virus (HIV) infection. Infection 1991; 19: 140–5
Meyer JL, Crosby RA, Whittington WL, et al. The psychosocial impact ofserological herpes simplex type 2 testing in an urban HIV clinic. Sex TransmInfect 2005; 81: 309–15
Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of herpes simplexvirus in human immunodeficiency virus-infected women: effects of hormonalcontraception, pregnancy, and vitamin A deficiency. J Infect Dis 2000; 181:58–63
Mbizvo M, Mashu A, Chipato T, et al. Trends in HIV-1 and HIV-2 prevalence andrisk factors in pregnant women in Harare, Zimbabwe. Cent Afr J Med 1996; 42:14–21
Wald A, Link K. Risk of human immunodeficiency virus in herpes simplex virustype 2-seropositive persons: a meta-analysis. J Infect Dis 2002; 185: 45–52
Corey L, Wald A, Celum CL, et al. The effects of herpes simplex virus-2 on HIV-1acquisition and transmission: a review of two overlapping epidemics. J AcquirImmune Defic Syndr 2004; 35: 435–45
Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission percoital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai,Uganda. Lancet 2001; 357: 1149–53
Schacker T. The role of HSV in the transmission and progression of HIV. Herpes2001 Jul; 8(2): 46–9
Augenbraun M, Feldman J, Chirgwin K, et al. Increased genital shedding of herpessimplex virus type 2 in HIV-seropositive women. Ann Intern Med 1995; 123:845–7
Schacker T, Zeh J, Hu HL, et al. Frequency of symptomatic and asymptomaticherpes simplex virus type 2 reactivations among human immunodeficiencyvirus-infected men. J Infect Dis 1998; 178: 1616–22
Ioannidis J, Collier AC, Cooper DA, et al. Clinical efficacy of high-dose acyclovirin patients with human immunodeficiency virus infection: a meta-analysis ofrandomized individual patient data. J Infect Dis 1998; 178: 349–59
Schacker T, Zeh J, Hu H, et al. Changes in plasma human immunodeficiency virustype 1 RNA associated with herpes simplex virus reactivation and suppression.J Infect Dis 2002; 186: 1718–25
Levin MJ, Bacon TH, Leary JJ. Resistance of herpes simplex virus infections tonucleoside analogues in HIV-infected patients. Clin Infect Dis 2004 Nov 1; 39Suppl. 5: S248–57
Wright PW, Hoesley CJ, Squires KE, et al. A prospective study of genital herpessimplex virus type 2 infection in human immunodeficiency virus type 1(HIV-1)-seropositive women: correlations with CD4 cell count and plasmaHIV-1 RNA level. Clin Infect Dis 2003 Jan 15; 36(2): 207–11
Danve-Szatanek C, Aymard M, Thouvenot D, et al. Surveillance network forherpes simplex virus resistance to antiviral drugs: 3-year follow-up. J ClinMicrobiol 2004; 42(1): 242–9
Acknowledgements
This article was supported in part by grants from the National Institutes of Health (grant T32 AI-07140 to Dr Strick and grant AI-30731 to Dr Wald). The authors would like to thank Meei-Li Huang for her contribution to this article.
The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strick, L.B., Wald, A. Diagnostics for Herpes Simplex Virus. Mol Diag Ther 10, 17–28 (2006). https://doi.org/10.1007/BF03256439
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256439