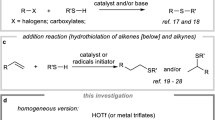
Abstract
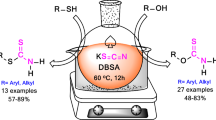
An environmentally benign and efficient process for the preparation of thioethers was developed by simple and practical reactions of alkyl halides and thiols in water in the presence of K2CO3 or Et3N in very high yields. The reaction of aryl, alkyl, aliphatic and hindered thiols with various alkyl halides gave the corresponding products with significant advantages such as high conversions, short reaction time, mild reaction conditions, and low cost, simple workup with good to quantitative yields.
Similar content being viewed by others
References
C.-J. Li, Chem. Rev. 105 (2005) 3095
H. Firouzabadi, N. Iranpoor, F. Nowrouzi, Chem. Commun. (2005) 789
M.C. Pirrung, K.D. Sarma, J. Am. Chem. Soc. 126 (2004) 444
H. Firouzabadia, N. Iranpoor, H. Alinezhad, J. Iran. Chem. Soc. 6 (2009) 177
A.A. Jafari, F. Moradgholi, F. Tamaddon, J. Iran. Chem. Soc. 6 (2009) 588
H. Zali Boeini, J. Iran. Chem. Soc. 6 (2009) 547
M. Bararjanian, S. Balalaie, B. Movassagh, A.M. Amani, J. Iran. Chem. Soc. 6 (2009) 436
N. Azizi, E. Akbari, M.R. Saidi, J. Iran. Chem. Soc. 6 (2009) 165.
H. Firouzabadi, A. Jamalian, J. Sulfur Chem. 28 (2007) 631
R.J. Cremlyn, Introduction to Organo-Sulfur Chemistry, Wiley & Sons, New York, 1996
M.D. McReynolds, J.M. Doughtery, P.R. Hanson, Chem. Rev. 104 (2004) 2239.
J.Y. Hwang, Y.-D. Gong, J. Comb. Chem. 8 (2006) 297.
G. Solladie, Synthesis (1981) 185
G.H. Posner, in: S. Patai, Z. Rappoport, C.J.M. Sterling (Eds.), The Chemistry of Sulfones and Sulfoxides, Chap. 16, Wiley, Chichester, 1988, pp. 823–849.
L.Y. Goh, M.E. Teo, S.B. Khoo, W.K. Leong, J.J. Vittal, J. Organomet. Chem. 664 (2002) 161
C.A. Christensen, M. Meldal, J. Comb. Chem. 9 (2007) 79.
R.N. Salvatore, R.A. Smith, A.K. Nischwitz T. Gavin, Tetrahedron Lett. 46 (2005) 8931; au[b)_S. Patai, The Chemistry of the Functional Groups-The Chemistry of the Thiol Group, Wiley, London, UK, 1974, p. 669
J.F. Boscato, J.M. Catala, E. Franta, J. Brossas, Tetrahedron Lett. 21 (1980) 1519
F.J.A. Hundscheid, V.K. Tandon, P.H.A.M. Rouwette, A.M. Van Leusen, Tetrahedron 43 (1987) 5073
J. Malmstrom, V. Gupta, L. Engman, J. Org. Chem. 63 (1998) 3318
P. Blanchard, B. Jousselme, P. Frere, J. Roncali, J. Org. Chem. 67 (2002) 3961
D. Landini, F. Rolla, Synthesis (1974) 496
J.M. Khurana, P.K. Sahoo, Synth. Commun. 22 (1992) 1691
P.C. Herradura, K.A. Pendola, R.K. Guy, Org. Lett. 2 (2000) 2019
N. Taniguchi, J. Org. Chem. 69 (2004) 6904
M.T. Martin, A.M. Thomas, D.G. York, Tetrahedron Lett. 43 (2002) 2145
N.A. Sasaki, C. Hashimoto, P. Potier, Tetrahedron Lett. 28 (1987) 6069.
D.P. Curran, In Comprehensive Organic Synthesis, Vol. 4
H. Firouzabadi, M. Jafarpour, J. Iran. Chem. Soc. 5 (2008) 159
B.M. Trost, I. Fleming (Eds.), Pergamon, New York, 715 (1991)
K. Griesbaum, Angew. Chem., Int. Ed. Engl. 9 (1970) 273
C.G. Screttas, M.J. Micha-Screttas, J. Org. Chem. 44 (1979) 713
M. Belley, R. Zamboni, J. Org. Chem. 54 (1989) 1230
P. Kumar, R.K. Pandey, V.R. Hegde, Synlett (1999) 1921
S. Kanagasabathy, A. Sudalai, B.C. Benicewicz, Tetrahedron Lett. 42 (2001) 3791.
H. Firouzabadi, N. Iranpoor, A.A. Jafari, Synlett (2005) 299
H. Firouzabadi, N. Iranpoor, A.A. Jafari, Adv. Synth. Cat. 347 (2005) 655
H. Firouzabadi, N. Iranpoor, M. Jafarpour, A.M. Ghaderi, J. Mol. Catal. A. Chem. 249 (2006) 98
G.L. Khatik, R. Kumar, A.K. Chakraborti, Org. Lett. 8 (2006) 2433.
H. Firouzabadi, N. Iranpoor, A.A. Jafari, J. Mol. Cat. A: Chem. 250 (2006) 237
F. Fringuelli, F. Pizzo, S. Tortoioli, L. Vaccaro, Adv. Synth. Catal. 344 (2002) 379
B.P. Bandgar, A.V. Patil, O.S. Chavan, V.T. Kamble, Catal. Commun. 8 (2007) 1065.
B.C. Ranu, T. Mandal, Tetrahedron Lett. 47 (2006) 6911
B.C. Ranu, T. Mandal, Synlett (2007) 925
B.C. Ranu, R. Jana, Adv. Synth. Catal. 347 (2005) 1811
H. Firouzabadi, N. Iranpoor, M. Jafarpour, Tetrahedron Lett. 47 (2006) 93
H. Firouzabadi, N. Iranpoor, M. Jafarpour, J. Sulfur Chem. 26 (2005) 313
H. Firouzabadi, N. Iranpoor, A.A. Jafari, Tetrahedron Lett. 46 (2005) 2683.
N. Azizi, F. Aryanasab, L. Torkiyan, A. Ziyaei, M.R. Saidi, J. Org. Chem. 71 (2006) 3634
N. Azizi, L. Torkiyan, M.R. Saidi, Org. Lett. 8 (2006) 2079
N. Azizi, M.R. Saidi, Org. Lett. 7 (2005) 3649
N. Azizi, M.R. Saidi, Tetrahedron 63 (2007) 888
N. Azizi, F. Aryanasab, M.R. Saidi, Org. Biomol. Chem. (2006) 4275.
N. Azizi, M.R. Saidi, Organometallics 23 (2004) 1457
N. Azizi, M.R. Saidi, Tetrahedron 60 (2004) 383
N. Azizi, F. Rajabi, M.R. Saidi, Tetrahedron Lett. 45 (2004) 9233
B. Mirmashhori, N. Azizi, M.R. Saidi, J. Mole. Catal. A: Chem. 247 (2006) 159
N. Azizi, R. Yousefi, M.R. Saidi, J. Organomet. Chem. 691 (2006) 817
N. Azizi, M.R. Saidi, Catal. Commun. 7 (2006) 224
N. Azizi, F. Aryanasab, M.R. Saidi, Synlett (2007) 1239.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azizi, N., Khajeh Amiri, A., Bolourtchian, M. et al. A green and highly efficient alkylation of thiols in water. JICS 6, 749–753 (2009). https://doi.org/10.1007/BF03246165
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03246165