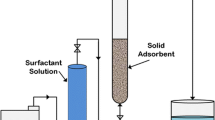
Abstract
The adsorption of Triton X-100 in aqueous solution on the granite sand has been investigated to evaluate its ability as an adsorbent. Various parameters such as agitation time, adsorbent dose, adsorbent size, initial concentration of adsorbate, pH, temperature, and effect of interference ions were studied on the laboratory scale to establish optimum conditions for the removal of TX-100 from the effluents of different industries. Isotherm data were analyzed for possible agreement with the Langmuir and Frendlich adsorption isotherm equations. The first order rate equation by Lagergren was tested on the kinetic data. The rate of adsorption was conformed a pseudo first order kinetics with good correlation coefficient. The value of activation energy of sorption (Ea) was obtained as 44.6 kJ mol−1. Results showed that granite sand exhibit reasonably good surfactant removals for nonionic types. The possible role of the adsorbent in a chromatographic column was also worked out.
Similar content being viewed by others
References
World Health Organization (W.H.O) International Standard for Drinking Water, 1971.
R.D. Swisher, Surfactant Biodegradation, (2nd edition) Marcel Dekker, New York, 1987.
V. Mezzanotte, F. Castiglioni, R. Todeschini, M. Pavan, Bioresource Technol. 87 (2003) 87.
J.S. Metthew, N.J. Malcolm, Biochim. Biophys. Acta 1508 (2000) 235.
D. Abdelhafidh, H. Naima, H. Ilem, S. Sami, Process Biochem. 38 (2003) 1245.
Zeki Aktas, Turk. J. Chem. 25 (2001) 311.
J.M. Douillard, S. Pougnet, B. Faucompre, S. Partyka, J. Colloid Interface Sci. 154 (1992) 113.
S.G. Dai, L. Dong, Z. Wang, Zhongguo Huanjing Kexue 19 (1999) 392.
A.A. Atia, N.R.E. Radwan, Adsorpt. Sci. Technol. 15 (1997) 619.
A.M. Blokhus, H. Hoeiland, M. Gierfde, J. Colloid Interface Sci. 179 (1996) 625.
S.W. Musselman, S. Chander, J. Colloid Interface Sci. 256 (2002) 91.
H.U. Foersterling, R. Khanikowski, Tenside Surfactants Deterg. 30 (1993) 48.
V.L. Alexeev, P. IIekti, J. Persello, J. Lambard, Langmuir 12 (1996) 2392.
J. Seidel, Thermochim. Acta 229 (1993) 257.
Hu, Wenzhi, P.R. Haddad, Anal. Commun. 35 (1998) 191.
L. Fang, M.J. Rosen, J. Colloid Interface Sci. 224 (2000) 265.
M.N. Shalaby, A.A. El-Feky, J. Dispersion Sci. Technol. 20 (1999) 1389.
P. D. Purakayastha, A. Pal, M. Bandyopadhyay, J. Environ. Sci. & Health 37 (2001) 925.
M.S. Akhter, S.M. Al-Alawi, Colloids Surf. A 196 (2002) 163.
M.S. Akhter, S.M. Al-Alawi, Colloids Surf. A 219 (2003) 281.
Y.S. Ho, C.C. Chiang, Adsorption 7 (2001) 139.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasiruddin Khan, M., Zareen, U. Adsorptive removal of non-ionic surfactants from water using granite sand. JICS 1, 152–158 (2004). https://doi.org/10.1007/BF03246108
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03246108