Abstract
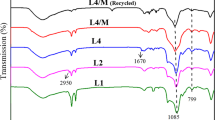
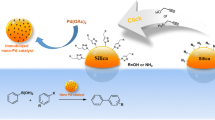
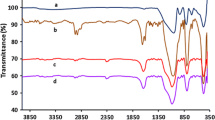
Cobalt(II) Schiff base functionalized mesoporous silica was synthesized from covalent attachment via the introduction of Co(OAc)2 to salicylaldimine functionalized mesoporous silica. The catalyst proved to be chemoselective one for the acetalization of aldehydes to the corresponding acetals in alcohol. The immobilized catalyst can be easily recovered and reused for at least ten reaction cycles without significant loss of its catalytic activity.
Similar content being viewed by others
References
S. Tamagaki, R.J. Card, D.C. Neckers, J. Am. Chem. Soc. 100 (1978) 6635.
A.P. Wight, M.E. Davis, Chem. Rev. 102 (2002) 3569.
A. Taguchi, F. Schuth, Micropor. Mesopor. Mater. 77 (2005) 1.
A. Corma, H. Garcia, Chem. Rev. 102 (2002) 3837.
D.E. DeVos, M. Dams, B.F. Sels, P.A. Jacobs, Chem. Rev. 102 (2002) 3615.
L. Yin, J. Liebscher, Chem. Rev. 107 (2007) 133.
N.E. Leadbeater, M. Marco, Chem. Rev. 102 (2002) 3217.
L. Canali, D.C. Sherrington, Chem. Rev. 81 (1981) 557.
Q.-H. Fan, Y.-M. Li, A.S.C. Chan, Chem. Rev. 102 (2002) 3385.
C.E. Song, S. Lee, Chem. Rev. 102 (2002) 3495.
P. Sutra, D. Brunel, Chem. Commun. (1996) 2485.
R.J.P. Corriu, E. Lancelle-Beltran, A. Mehdi, C. Reye, S. Brandes, R. Guilard, Chem. Mater. 15 (2003) 3152.
T.W. Greene, P.G.M. Wuts, Protective Groups in Organic Synthesis, 3th ed., Wiley, New York, 1999.
P.J. Kocienski, Protecting Groups, Thieme, New York, 1994.
H.J.E. Loewnthal, in: J.F.W. McOmie (Ed.), Protective Groups in Organic Chemistry, Plenum Press, London, 1973.
J.R. Hanson, Protecting Groups in Organic Synthesis, 1st ed., Blackwell Science, Inc: Malden, Mass., 1999.
J.N. Shepherd, D.C. Myles, Org. Lett. 5 (2003) 1027.
J.K. Whitesell, Chem. Rev. 89 (1989) 1581.
D. Seebach, R. Imwinkelried, T. Weber, in: R. Schffold (Ed.), Modern Synthetic Methods, Vol. 4, Springer-Verlag, Berlin, 1986, pp. 125–259.
J. Otera, T. Mizutani, H. Nazaki, Organometallics 8 (1989) 2063.
B. Karimi, B. Golshani, Synthesis (2002) 784.
S. Vetrivel, A. Pandurangan, J. Mol. Catal. A 217 (2004) 165.
C.T. Chen, S.S. Weng, J.Q. Kao, C.C. Lin, M.D. Jan, Org. Lett. 7 (2005) 3343.
B. Karimi, H. Seradj, G.R. Ebrahimian, Synlett (1999) 1456.
A.J.F. Meskens, Synthesis (1981) 501.
R. Gopinath, S.J. Haque, B.K. Patel, J. Org. Chem. 67 (2002) 5842.
B. Perio, M.J. Dozians, P. Jacquault, J. Hamelin, Tetrahedron Lett. 38 (1997) 7867.
S.H. Lee, J.H. Lee, C.M. Yoon, Tetrahedron Lett. 43 (2002) 2699.
J. Tateiwa, H. Horiuchi, S. Uemura, J. Org. Chem. 60 (1995) 4039.
S. Velusamy, T. Punniyamurthy, Tetrahedron Lett. 45 (2004) 4917.
B.M. Smith, A.E. Graham, Tetrahedron Lett. 47 (2006) 9317.
B.T. Gregg, K.C. Golden, J.F. Quinn, Tetrahedron 64 (2008) 3287.
N.M. Leonard, M.C. Oswald, D.A. Freiberg, B.A. Nattier, R.C. Smith, R.S. Mohan, J. Org. Chem. 67 (2002) 5202.
J. Otera, N. Dan-Oh, H. Nozaki, Tetrahehron 48 (1992) 1449.
H. Firouzabadi, N. Iranpoor, B. Karimi, Synth. Commun. 29 (1999) 2255.
B. Karimi, M. Ghoreishi-Nezhad, J. Mol. Catal. A 277 (2007) 262.
I. Rodriguez, M.J. Climent, S. Iborra, V. Fornes, A. Corma, J. Catal. 192 (2000) 441.
M.V. Joshi, C.S. Narasimhan, J. Catal. 128 (1991) 63.
M.W.C. Robinson, A.E. Graham, Tetrahedron Lett. 48 (2007) 4727.
W.M. Van Rhijn, D.E. DeVor, B.F. Sels, W.D. Bossaert, P.A. Jacobs, Chem. Commun. (1998) 317.
D. Margolese, J.A. Melero, S.C. Christiansen, B.F. Chmelka, G.D. Stucky, Chem. Mater. 12 (2000) 2448.
K. Wilson, A. Renson, J.H. Clark, Pure Appl. Chem. 72 (2000) 1313.
M.H. Lim, C.F. Blanford, A. Stein, Chem. Mater. 10 (1998) 467.
Q. Bao, K. Qiao, D. Tomida, C. Yokoyama, Catal. Commun. 10 (2009) 1625.
C. Huo, T.H. Chan, Adv. Synth. Catal. 351 (2009) 1933.
F. Rajabi, J.H. Clark, B. Karimi, D.J. Macquarrie, Org. Biomol. Chem. 3 (2005) 725.
F. Rajabi, B. Karimi, J. Mol. Catal. A: Chem. 232 (2005) 95.
I.C. Chisem, J. Rafelt, M.T. Shieh, J. Chisem, J.H. Clark, R. Jachuck, D. Macquarrie, C. Ramshaw, K. Scott, Chem. Commun. (1998) 1949.
F. Rajabi, Tetrahedron Lett. 50 (2009) 395.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rajabi, F. Cobalt(II) schiff base functionalized mesoporous silica as an efficient and recyclable chemoselective acetalization catalyst. JICS 7, 695–701 (2010). https://doi.org/10.1007/BF03246059
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03246059