Abstract
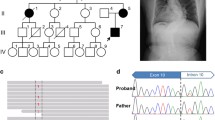
Osteogenesis imperfecta (OI) is a bone dysplasia caused by mutations in theCOL1A1 andCOL1A2 genes. Although the condition has been intensely studied for over 25 years and recently over 800 novel mutations have been published, the relation between the location of mutations and clinical manifestation is poorly understood. Here we report missense mutations inCOL1A1 of several OI patients. Two novel mutations were found in the D1 period. One caused a substitution of glycine 200 by valine at the N-terminus of D1 in OI type I/IV, lowering collagen stability by 50% at 34°C. The other one was a substitution of valine 349 by phenylalanine at the C-terminus of D1 in OI type I, lowering collagen stability at 37.5°C. Two other mutations, reported before, changed amino residues in D4. One was a lethal substitution changing glycine 866 to serine in genetically identical twins with OI type II. That mutated amino acid was near the border of D3 and D4. The second mutation changed glycine 1040 to serine located at the border of D4 and D0.4, in a proband manifesting OI type III, and lowered collagen stability at 39°C (2°C lower than normal). Our results confirm the hypothesis on a critical role of the D1 and D4 regions in stabilization of the collagen triple-helix. The defect in D1 seemed to produce a milder clinical type of OI, whereas the defect in the C-terminal end of collagen type caused the more severe or lethal types of OI.
Similar content being viewed by others
References
Arnold WV, Fertala A, Sieron AL, Bachinger H-P, Mechling D, Prockop DJ, 1998. Recombinant procollagen II. Deletion of D-period segments identifies sequences that are required for helix stabilization and generates a temperature-sensitive N-proteinase cleavage site. J Biol Chem 273: 31822–31828.
Benusiene E, Kucinskas V, 2003. COL1A1 mutation analysis in Lithuanian patients with osteogenesis imperfecta. J Appl Genet 44: 95–102.
Berg RA, Prockop DJ, 1973. The thermal transition of a nonhydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Bioechem Bioph Res Co 52: 115–120.
Bruckner P, Prockop DJ, 1981. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem 110: 360–368.
Byers PH, 1993. Osteogenesis imperfecta. In: Royce PM, Steinmann B, eds. Connective tissue and its heritable disorders; 1st ed. New York: Wiley-Liss, Inc: 317–351.
Byers PH, Wallis GA, Willing MC, 1991. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet 28: 433–442.
Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, et al. 2004. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science 303: 1198–1201.
Cheah KS, 1985. Collagen genes and inherited connective tissue disease. Biochem J 229: 287–303.
Colige A, Li S-W, Sieron AL, Nusgens BV, Prockop DJ, Lapiere CM, 1997. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc Nat Acad Sci USA 94: 2374–2379.
Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, et al. 1999. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet 65: 308–317.
Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD, 2002. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem 277: 4223–4231.
Fertala A, Sieron AL, Ganguly A, Li S-W, Ala-Kokko L, Anumula KR, Prockop DJ, 1994. Synthesis of re-combinant human procollagen II in stably transfected tumor cell line (HT-1080). Biochem J 298: 31–37.
Fertala A, Han WB, Ko FK, 2001. Mapping critical sites in collagen II for rational design of gene-engineered proteins for cell-supporting materials. J Biomed Mater Res 57: 48–58.
Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, et al. 2000. Type V osteogenesis imperfecta; a new form of brittle bone disease. J Bone Miner Res 15: 1650–1658.
Glorieux FH, Ward LM, Rauch F, Lalic L, Roughley PJ, Travers R, 2002. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res 17: 30–38.
Kuivaniemi H, Tromp G, Prockop DJ, 1991. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J 5: 2052–2060.
Lund AM, Skovby F, Schwartz M, 1997. Serine for glycine substitutions in the C-terminal third of the alpha 1(I) chain of collagen I in five patients with nonlethal osteogenesis imperfecta. Hum Mutat 9: 378–82.
Lund AM, Astrom E, Soderhall S, Schwartz M, Skovby F, 1999. Osteogenesis imperfecta: mosaicism and refinement of the genotype-phenotype map in OI type III. Hum Mutat Mutations in brief no. 242. Online. 13: 503.
Majsterek I, McAdams E, Adachi E, Dhume ST, Fertala A, 2003. Prospects and limitations of the rational engineering of fibrillar collagens. Protein Sci 12: 2063–2072.
Malfait F, De Coster P, Hausser I, van Essen AJ, Franck P, Colige A, 2004. The natural history, including orofacial features of three patients with Ehlers-Danlos syndrome, dermatosparaxis type (EDS type VIIC). Am J Med Genet A 131: 18–28.
Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, 2007. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat 28: 209–221.
Namikawa C, Suzumori K, Fukushima Y, Sasaki M, Hata A, 1995. Recurrence of osteogenesis imperfecta because of paternal mosaicism: Gly862Ser substitution in a type I collagen gene (COL1A1). Hum Genet 95: 666–670.
Olsen AS, Geddis AE, Prockop DJ, 1991. High levels of expression of a minigene version of the human pro alpha 1 (I) collagen gene in stably transfected mouse fibroblasts. Effects of deleting putative regulatory sequences in the first intron. J Biol Chem 266: 1117–1121.
Piez KA, Gross J, 1959. The amino acid composition and morphology of some invertebrate and vertebrate collagens. Biochim Biophys Acta 34: 24–39.
Prockop DJ, Kivirikko KI, 1995. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64: 403–434.
Prockop DJ, Chu ML, 1993. Collagen: gene structure. [In:] Royce PM, Steinmann B, ed. Connective tissue and its heritable disorders; 1st ed. New York: Wiley-Liss, Inc: 149–167.
Raghunath M, Bruckner P, Steinmann B, 1994. Delayed triple helix formation of mutant collagen from patients with osteogenesis imperfecta. J Mol Biol 236: 940–949.
Roughley PJ, Rauch F, Glorieux FH, 2003. Osteogenesis imperfecta — clinical and molecular diversity. Eur Cell Mater 5: 41–47.
Sieron AL, Fertala A, Ala-Kokko L, Prockop DJ, 1993. Deletion of a large domain in recombinant human procollagen II does not alter the thermal stability of the triple helix. J Biol Chem 268: 21232–21237.
Sieron AL, Louneva N, Fertala A, 2002. Site-specific interaction of bone morphogenetic protein with procollagen II. Cytokine 18: 214–221.
Sillence DO, Senn A, Danks DM. 1979. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16: 101–116.
Slayton RL, Deschenes SP, Willing MC, 2000. Nonsense mutations in theCOL1A1 gene preferentially reduce nuclear levels of mRNA but not hnRNA in osteogenesis imperfecta type I cell strains. Matrix Biol 19: 1–9.
Steplewski A, Ito H, Rucker E, Brittingham RJ, Alabyeva T, Gandhi M, et al. 2004. Position of single amino acid substitutions in the collagen triple helix determines their effect on structure of collagen fibrils. J Struct Biol 148: 326–337.
Steplewski A, Majsterek I, McAdams E, Rucker E, Brittingham RJ, Ito H, Hirai K, et al. 2004a. Thermostability gradient in the collagen triple helix reveals its multi-domain structure. J Mol Biol 338: 989–998.
Virdi AS, Loughlin JA, Irven CM, Goodship J, Sykes BC, 1994. Mutation screening by a combination of biotin-SSCP and direct sequencing. Hum Genet 93: 287–290.
Ward LM, Rauch F, Travers R, Chabot G, Azouz EM, Lalic L, et al. 2002. Osteogenesis imperfecta type VII: an autosomal recessive form of brittle bone disease. Bone, 31: 12–18.
Willing MC, Deschenes SP, Scott DA, Byers PH, Slayton RL, Pitts SH, et al. 1994. Osteogenesis imperfecta type I: molecular heterogeneity for COL1A1 null alleles. Am J Hum Genet 55: 638–647.
Zhuang J, Tromp G, Kuivaniemi H, Castells S, Bugge M, Prockop DJ. 1996. Direct sequencing of PCR products derived from cDNAs for the pro alpha 1 and pro alpha 2 chains of type I procollagen as a screening method to detect mutations in patients with osteogenesis imperfecta. Hum Mutat 7: 89–99.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Witecka, J., Auguściak-Duma, A.M., Kruczek, A. et al. Two novelCOL1A1 mutations in patients with osteogenesis imperfecta (OI) affect the stability of the collagen type I triple-helix. J Appl Genet 49, 283–295 (2008). https://doi.org/10.1007/BF03195625
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03195625