Summary
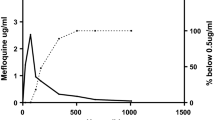
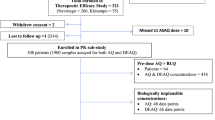
A new fixed-dose combination of artesunate (AS) plus amodiaquine (AQ) (TRIMALACT®) was recently developed for the treatment of uncomplicatedfalciparum malaria. The originality of this combination lies in its galenic formulation which consists of a threelayer tablet with two layers containing each of the active ingredients, i.e. AS and AQ, and these are separated by a middle layer containing an antioxidant compound. To evaluate the efficacy and tolerability of this combination, adults with uncomplicated malaria received three administrations of two tablets (100:300 mg AS/AQ) in a 24-h interval, in Democratic Republic of Congo. Parasitemia and fever were measured and the plasma levels of parent compounds and metabolites [dihydroartemisinin (DHA) and monodeselhylamodiaquine (MdAQ)] were determined by high-performance liquid chromatography. In addition, we determined the prevalence of molecular markers of resistance to chloroquine (CQ) and sulfadoxine/pyrimethamine (SP). The AS/AQ combination TRIMALACT® demonstrated a good efficacy resulting in an excellent clinical and parasitological response rate of 100% after correction for PCR results. Treatment regimen was well tolerated. The main disposition parameters to AS+AQ were: for DHA, AUC = 632 + 475 ng h/ml and Cmax= 432 ± 325 ng/ml, and for MdAQ = 14268 ± 4114 ng h/ml and Cmax= 336 ± 225 ng/ml (mean ± standard deviation). Parasite genotyping show high frequencies of molecular SP- and CQ-resistance markers with more 80% of the samples showing more than three mutations linked to SP resistance and 93.48% carrying parasite with the CQ-resistant haplotype. This study shows that the AS/AQ combination TRIMALACT® is safe and effective in the treatment of highly drug-resistantfalciparum malaria.
Similar content being viewed by others
References
World Health Organisation (2008). World Malaria Report 2008 WHO/HTM/MAL/2008.1. World Health Organisation, Geneva, Switzerland, http://www.who.int/nialaria/wmr2008/.
World Health Organisation (2006a). Guidelines for the treatment of malaria. World Health Organisation, Geneva, Switzerland WHO/HTM/MAI72006.1108. http//www.who.int/malaria/docs/TreatmentGuidelines2006.pd.
Sirima S.B., Gansané A. (2007). Artesunate-amodiaquine for the treatment of uncomplicated malaria. Expert Opin. Investig. Drugs, 16, 1079–1085.
World Health Organisation (2006b). Facts on ACTs (artemisinin-based combinations). World Health Organisation, Geneva, Switzerland http://www.rollbackmalaria.org/cmc_upload/0/000/015/364/RBMInf osheet_9/pdf.
Wembonyama S., Mpaka S., Tshilolo L. (2007). Medicine and health in the Democratic Republic of Congo: from independence to the third republic, Med. Trop., 67, 447–457.
Kazadi W., Sexton J.D., Bigonsa M., W’Okanga B., Way M. (2004) Malaria in primary school children and infants in Kinshasa, Democratic Republic of the Congo: surveys from the 1980S and 2000. Am. J. Trop. Med., 71, 97–102.
Coene J. (1993). Malaria in urban and rural Kinshasa: the entomological input. Med. Vet. Entomol., 7, 127–137.
Bard B., Carrupt P.-A. (2008) Dissolution study of TRIMALACT. Expertise Report from the LTC-Pharmacochemistry Department Pharmaceutical Sciences Division, Geneva and Lausanne Universities.
Vo T.K., Bigot P., Gazin P., Sinou V., De Pina J.J., Huynh D.C., Fumoux F., Parzy D. (2007). Evaluation of a real-time PCR assay for malaria diagnosis in patients from Vietnam and in returned travellers. Trans. R. Soc. Trop. Med. Hyg, 101:422–428.
Reeder J.C., Rieckmann K.H., Genton B., Lorry K., Wines B., Cowman A.F. (1996). Point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes andin vitro susceptibility to pyrimethamine and cycloguanil ofPlasmodium falciparum isolates from Papua New Guinea. Am. J. Trop. Med. Hyg., 55, 209–213.
Parzy D., Doerig G., Pradines B., Rico A., Fusai T., Doury J.C. (1997). Proguanil resistance inPlasmodium falciparum African isolates: assessment by mutation-specific polymerase chain reaction andin vitro susceptibility testing. Am J. Trop. Med. Hyg., 57, 646–650.
Djimbé A., Doumbo O.K., Cortese J.F., Kayentao K., Doumbo S., Diourté Y., Dicko A., Su X.Z., Nomura T., Fidock D.A., Wellems T.E., Plowe C.V., Coulibaly D. (2001). A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med., 344, 299–302.
Bogreau H., Renaud F., Bouchiba H., Durand P., Assi S.-B., Henry M.-C., Garnotal E., Pradines B., Fusai T., Wade B., Adehossi E., Parola P., Ali Kamil M., Puijalon O., Rogier C. (2006). Genetic diversity and structur of AfricanPlasmodium falciparum populations in urban and rural areas. Am. J. Trop. Med Hyg., 74, 953–959.
Su X., Wellems T.E. (1996). Toward a high-resolutionPlasmodium falciparum linkage map: polymorphic markers from hundreds of simple sequence repeats. Genomics, 33, 430–444.
Anderson T.J., Su. X.Z., Bockarie M., Lagog M., Day K.P. (1999). Twelve microsatellite markers for characterization ofPlasmodium falciparum from finger-prick blood samples. Parasitology, 119, 113–125.
Mulumba P.M. (2002).Situation épidémiologique du paludisme en République Démocratique du Congo. Congo Médical, 5, 431–435.
Delacollette C., Embonga B., Malengreau M. (1983). Response to chloroquine of infections with Plasmodium falciparum in the Kivu region. Ann. Soc. Belg. Med. Trop., 53, 171–173.
World Health Organisation (2005). Susceptibility of Plasmodium falciparum to Antimalarial Drugs, Report on Global Monitoring 1996–2004. WHO/HTM/MAL/2005.1103. World Health Organisation, Geneva, Switzerland
Cohuet S., Bonnet M., Van Herp M., Van Overmeir C., D’Alessandro U., Gluthmann J.-P. (2006). Short report: moleclar markers associated with Plasmodium falciparum resistance to sulfadoxine-pyrimethamine in the Democratic Republic of Congo, Am. J. Trop. Med. Hyg., 75, 152–154.
Swarthout T.D., van den Broek I.V., Kayembe G., Montgomery J., Pota H., Roper C. (2006). Artesunate + amodiaquine and artesunate + sulfadoxine-pyrimethamine for treatment of uncomplicated malaria in Democratic Republic of Congo: a clinical trial with determination of sulfadoxine and pyrimethamine-resistant haplotypes. Trop. Med. Int. Health, 11, 1503–1511.
Alker A.P., Kazadi W.M., Kutelemeni A.K., Bioland P.B., Tshefu A.K., Meshnick S.R. (2008).dhfr and dhps genotype and sulfadoxine-pyrimethamine treatment failure in children with falciparum malaria in the Democratic Republic of Congo. Trop. Med. Int. Health, 11, 1384–1391.
Peters P.J., Thigpen M.C., Parise M.E., Newman R.D. (2007). Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug. Saf., 30,481–501.
Omar S.A. Adagu I.S., Wharhust D.C. (2001). Can pretreatment screening for dhps and dhfr point mutations in Plasmodium falciparum infections be used to predict sulfadoxine-pyrimethamine treatment failure? Trans. R. Soc. Trop. Med. Hyg., 95, 315–319.
Kublin J.G., Dzinjalamala F.K., Kamwendo D.D., Malkin E.M., Cortese J.F., Martino L.M., Mukadam R.A., Rogerson S.J., Lescano A.G., Molyneux M.E., Winstanley P.A., Chimpeni P., Taylor T.E., Plowe C.V. (2002). Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis., 185, 380–388.
Staedke S.G., Sendagire H., Lamola S., Kamya M.R., Dorsey G., Rosenthal P.J. (2004). Relationship between age, molecular markers, and response to sulphadoxinepyrimethamine treatment in Kampala, Uganda. Trop. Med. Int. Health, 9, 624–629.
Gesase S., Gosling R.D., Hashim R., Ord R., Naidoo I., Madebe R., Mosha J.F., Joho A., Mandia V., Mrema H., Mapunda E., Savael Z., Lemnge M., Mosha F.W., Greenwood B., Roper C, Chandramohan D. 2009. High resistance ofPlasmodium falciparum to Sulphadoxine/Pyrimethamine in Northern Tanzania and the emergence of dhps resisatnce mutation at codon 581. PloS ONE, 4: e4569. doi: 10.1371 /journal.pone.0004569.
Wilson P.E., Kazadi W., Demster Kamwendo D., Mwapasa V., Purfield A., Meshnick S.R. (2005). Prevalence of pfert mutations in Congolese and Malawian Plasmodium falciparum isolates as determined by a new Taqman assay. Acta Trop., 93, 97–106.
Adjuik M., Agnamey P., Babiker A., Borrmann S., Brasseur P., Cisse M., Cobelens F., Diallo S., Faucher J.F., Garner P.,Gikunda S., Kremsner P.G., Krishna S., Lell B., Loolpapit M., Matsiegui P.-B., Missinou M.A., Mwanza J., Ntoumi F., Olliaro P., Osimbo P., Rezbach P., Some E., Taylor W.R.J. (2002). Amodiaquine-artesunate versus amodiaquine for uncomplicatedPlasmodium falciparum malaria in African children: a randomized, multicentre trial. Lancet, 359, 1365–1372.
Barennes H., Nagot N., Valea I., Koussoubé-Balima T., Ouedraogo A., Sanou T., Yé S. (2004). A randomized trial of amodiaquine and artesunate alone and in combination for the treatment of uncomplicated falciparum malaria in children from Burkina Faso. Trop. Med. Int. Health, 9, 438–444.
Sowunmi A., Fehintola F.A., Adedeji A.A., Gbotosho G.O., Tambo E., Fateye B.A., Happi T.C., Oduola A.M.J. (2005). Open randomized study of artesunate-amodiaquine vs. chloroquine-pyrimethamine-sulfadoxine for the treatment of uncomplicated Plasmodium falciparum malaria in Nigerian children. Trop. Med. Int. Health, 10, 1161–1170.
Benakis A., Paris M., Anh T.K., Binh T.Q., Plessas C.T., Plessas S.T. (1996). Pharmacokinetic study of dihydroartemisinin in malaria patients in Vietnam. Jpn J. Trop. Med. Hyg. 24. 71–76.
Benakis A., Paris M., Loutan L. Plessas C.T., Plessas S.T. (1997). Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am. J. Trop. Med. Hyg., 56, 17–23.
Halpaap B., Ndjavc M., Paris M., Benakis A., Kremsner P.G. (1998) Plasma levels of artesunate and dihydroartemisinin in children withPlasmodium falciparum malaria in Gabon after administration of 50-milligram artesunate suppositories. Am. J. Trop. Med. Hyg. 58, 365–368.
Sinou V., Taudon N., Mosnier J., Aglioni C., Bressolle F.M., Parzy D. (2008). Pharmacokinetics of artesunate in the domestic pig. J. Antimicrob.Chemother., 62, 566–574.
Pussard E., Verdier F., Paurisson F., Schemnann J.M., Le Bias J., Blayo M.C. (1987). Disposition of monodesethylarnodiaquine after a single oral dose of amodiaquine and three regimens for prophylaxis against Plasmodium falciparum malaria Eur. J. Clin. Pharmacol., 33, 409–414.
Winstanley P.A., Edwards G., Orme M.L., Bteekenridge A.M. (1987). Effect of dose size on amodiaquine phamiaaikinetics after aal administration. Eur. J. Clin. Phannacol., 33, 331–333.
Navaratnam V., Ramanathan S., Wahab M.S. A., Siew Hua G., Mansor S.M., Kiechel J.-R., Vaillant M., Taylor W.R.J., Olliaro P. (2009). Tolerability and pharmacokinetics of nonfixed and fixed combinations of artesunate and amodiaquine in Malaysian healthy normal volunteers. Eur. J. Clin. Pharmacol., 65, 809–821.
Orrell C., Little F., Smith P., Folb P., Taylor W., Olliaro P., Barnes K.I. (2008). Pharmacokinetics and tolerability of artesunate and amodiaquine alone and in combination in healthy volunteers. Eur. J. Clin. Pharmacol., 64, 683–690.
Author information
Authors and Affiliations
Additional information
Note: Parts of these results have been already presented as a Poster at the European Congress on Tropical Medicine and International Health, May 2007, Amsterdam, The Netherlands
Rights and permissions
About this article
Cite this article
Sinou, V., Maladca, L.T.M., Taudon, N. et al. Pharmacokinetics and pharmacodynamics of a new ACT formulation: Artesunate/Amodiaquine (TRIMALACT®) following oral administration in African malaria patients. Eur. J. Drug Metabol. Pharmacokinet. 34, 133–142 (2009). https://doi.org/10.1007/BF03191163
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03191163