Summary
In recent years, Artemisinin and particularly one of its derivatives — Artesunate (ART — has become an essential alternative for treatment of both uncomplicated and severe falciparum malaria in Asia and Africa as well.
Therefore, these compounds are still and inccreasingly in the focus of interest because of quick acting of this drug, is able to help even unconscious to overcome the malaria attack, when administered by injection. As an alternative, RECTOCAPS have been developed and their use is meanwhile well established. From earlier studies in children, suffering from Plasmodium falciparum malaria, we obtained a high level of DHART in the blood, but as expected also a rapid decline in the levels of both DHART and ART. A second administration of ART was additionally applied 4 hours after the first administration. DHART and ART plasma levels were found to last longer on an assumed therapeutic level than those obtained after one administration only. The fever clearance and the parasitemia reduction rates were found to be effective according to this dosing regimen. In view of these findings, we decided to conduct the actual described study by administering 200 mg of ART every 3 hours (0, 3, 6 and 9 h) by the rectal route.
Soft geiatine capsules (RECTOCAPS) containing 200 mg of ART GMP — type each (Artesunic acid) were administered by rectal route. Each patient received four RECTOCAPS capsules (4×200 mg of ART) over a 3 h period.
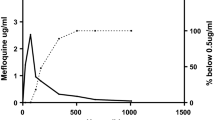
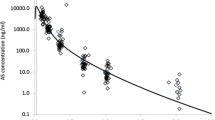
12 adult patients with uncomplicated malaria were selected. Age, weight, height, body temperature, parasite counts before treatment and their evolution until 96 h are determined. Blood samples were taken at short time intervals after starting with the first medication: 0, 30 min, 60 min, 3 h, 6 h, 9 h, 12 h, 24 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h and 108 h. The aliquots of all the blood samples were used for performing parasite counts. Plasma obtained following the traditional procedure was kept at −40°C until analysis. HPLC technique with electrochemical detector was used for quantification of ART and DHART. From the blood concentration values of ART and DHART, the following observation can be derived: the onset of action is observed within the first half hours, therapeutic levels of the drug obtained (89 μg/ml ART compared to 84 μg/ml DHART). The DHART levels are somewhat higher than those of ART (a peak concentration after 6 h starting medication of 151 μg/ml ART as compared to 276 μg/ml DHART). The variations as a function of frequency of DHART uptake are much less marked than those observed for ART. Another finding is that after the administration, some sort of a plateau of DHART and ART is built up, lasting at least from 9 to 12 hours with DHART level of about 190 μg/ml and ART of 90 μg/ml. In the case of single-dose administration, the levels of both compounds were below the detection threshold after three hours.
With regard to the parasite counts, although there were inter-individual variations, it should be noted that after 48 hours a high proportion of the patients (8 out of 12) was completely clear of parasites. Similar results were observed with regard to the body temperature (7 out of 12 returned to normal temperature 36 hours after starting the therapy). The findings of the study support the RECTOCAPS application principle resulting in effectiveness both for the velocity of drug uptake as well as for the height of plasma levels. Repeated administration of ART can extend the duration of therapeutic plasma levels of the drug.
Similar content being viewed by others
References
Batty, KT., Anh Thu, LT.,et al. (1998) A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicate falciparum malaria.Br. J. Clin. Pharmacol. 45, 123–129.
Bethell, D.B. Teja-Isavadharm, P.et al. (1997) Pharmacokinetics of oral artesunate in children with moderatory severe Plasmodium falciparum malaria.Trans. R. Soc. Trop. Med. Hygiene 91, 195–198.
Benakis, A., Paris, M., Plessas, C., Hien, T.T., Waller, D. and White, J.J. (1993) Pharmacokinetics of sodium artesunate after i.m. and i.v. administration. In:Joint Meeting of the American Society of Parasitologists and the American Society of Tropical Medicine and Hygiene. October 31–November 4, 1993, Atlanta, GA, USA.
Benakis A. Paris M., M. Loutan, L., Plessas, C.T. and Plessas, S.T. (1997) Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers.Am. J. Trop. Med. Hyg. 56(1), 17–23.
Halpaap, B., Ndjave, M., Paris, M., Benakis, A. and Kremsner, P.G. (1998) Plasma levels of artesunate and dihydroartemisinin in children withPlasmodium falciparum malaria in Gabon after administration of 50-milligram artesunate suppositories.Am. J. Trop. Med. Hyg. 58, 365–368.
Author information
Authors and Affiliations
Additional information
RECTOCAPS Plasmotrim ® MEPHA Ltd, Switzerland
Rights and permissions
About this article
Cite this article
Benakis, A., Binh, T.Q., Keundjian, A. et al. Pharmacokinetics/Pharmacodynamics findings after repeated Administration of ARTESUNATE thermostable suppositories (RECTOCAPS) in Vietnamese patients with uncomplicated malaria. European Journal of Drug Metabolism and Pharmacokinetics 31, 41–45 (2006). https://doi.org/10.1007/BF03190641
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190641