Summary
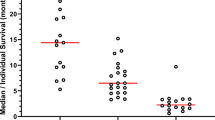
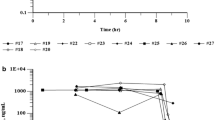
The concentration-time profiles of Doxorubicin (DOXO) from day 0 to day 21 after i.v. infusion of 25 or 30 mg/m2 doxorubicin HCl stealth liposomes (Caelyx®) were investigated in 9 patients receiving combination polychemotherapy with cyclophosphamide, vinorelbine and prednisone. Peak serum concentrations occurred from 0.04 to 4.0 days after infusion (mean tmax=1.79±1.55 d) with a mean cmax of 4595±2849 ng/ml. A total amount of 12.84±2.47 mg liposomal DOXO in the plasma volume (Vp=2794+537 ml) could be estimated at tmax (=27% of the mean dose of 47.6 mg). Stealth liposomes were eliminated slowly from the blood with a mean t1/2el of 1.9+0.5 days (MRT was 4.6+2.5 days).
AUClast values ranged from 8070 to 33446 ng/ml*d (mean 10987±9339 ng/ml*d). The low plasma clearance (Cltot=4681±2835 ml/day) and the small volume of distribution (Vz=11.7±6.3 l) suggested that stealth-liposomes were stable in the blood at least for 14 days. Polychemotherapy with Hyper-CCVP schedule did not alter the stability of stealth liposomes, but peak levels of DOXO seemed to be somewhat lower compared to regression analysis of literature data (cmax versus dosage range from 20 to 60 mg/m2). Due to clast occurring between day 12 to 18, no indices for an accumulation of the drug in the blood could be found, when liposomes were given every four weeks.
Similar content being viewed by others
References
Bielack S.S., Ertmann R., & Kempf-Bielack G. (1996): Impact of scheduling of toxicity and clinical efficacy of doxorubicin: what do we know in the mid-nineties? Eur. J. Cancer, 32A, 1652
SEQUUS Pharmaceutical Inc. Investigators Brochure (1997): “Doxorubicin encapsulated in stealth liposomes”, pp. 65–70
Allen T.M., Hansen C., Martin F., Redemann C., Yau-Yong A. (1991): Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half lives in-vivo. Biochim. Biophys. Acta, 1066, 29–36.
Huang S.K., Lee K.D., Hong K., Friend D.S., Papahadjopoulos D. (1992): Microscopic localisation of sterically stabilised liposomes in colon carcinoma bearing mice. Cancer Res., 52, 5135–43.
Huang S.K., Martin F.J., Jay G. (1993): Extravasation and transcytosis of liposomes in Kaposi’s sarcoma-like dermal lesions of transgenic mice bearing the HIC tat gene. Am. J. Pathol. 143, 10–14
Northfelt D.W., Kaplan L., Russell J., Volberding P.A., Martin F.J. Pharmacokinetics and tumour localisation of DOX-SL (STEALTH” liposomal doxorubicin) by comparison with Adriamycin in patients with AIDS and Kaposi’s sarcoma. In: Stealth Liposomes (Lasic D and Martin F, eds). Boca Raton, FL: CRC Press 1995: pp 257–66
Amantea M., Newman M.S., Sullivan T.M., Forrest A., Working P.K. (1999): Relationship of dose intensity to the induction of palmar-plantar erythro-dysesthia by pegylated liposomal doxorubicin in dogs. Human Exp. Toxicol., 18, 17–26.
Shipp M.A. (1993): A predictive model for aggressive Non-Hodgkin’s Lymphoma. New Engl. J. Med., 329, 987–994.
Czejka M.J., Georgopoulos A. (1988): High performance liquid chromato-graphic determination of adriblastin in human plasma, urine, saliva and liver punctuate by column switching for drug monitoring studies. J. Chromatogr. Biomed. Appl., 424, 182–188
Scientific Tables Geigy, Vol. III, Haematology and Human Genetics (Ciba Geigy Limited AG, Basle, Switzerland) 1983: pp 66–67
Czejka M.J., Schueller J., Linkesch W., Eder I., Zeleny U., Kraule C., Pernegg, C. Serum and tissue concentrations of doxorubicin in patients receiving i.v. mono- and polychemotherapy of encapsulated doxorubicin (stealth liposomes, Caelyx”). 28th Annual Symposium European Society of Clinical Pharmacy, Oct. 1999, Berlin, Abstract No. 60
Dinnendahl V., Tricke, U. (1996): Arzneistoffprofile, Basisinformation über arzneiliche Wirkstoffe, 11. Ergänzung, GOVI Verlag GmbH. Pharmazeutischer Verlag, Frankfurt, Germany
Schuler U., Ehninger G., Wagner, T. (1987): Repeated high dose cyclophosphamide administration in bone marrow transplantation; exposure to active metabolites. Cancer Chemother. Pharmacol., 3, 248
Evans, W.E., Crom, W.R. & Yee, G.C. (1980): Adriamycin pharmacokinetics in children. Proc. Am. Assoc. Cancer Res., 21, 176
Dodion, P., Riggs, C.E., Akman, S.R., Tamburini, J.M., Colvin, O.M. & Bachur N.R. (1984): Interactions between cyclophosphamide and adriamycin in rats. J. Pharmac. Exp. Ther., 229, 51–57
Dodion P., Akman S.R., Tamburini J.M., Riggs C.E., Colvin, O.M., Bachur N.R. (1986): Interaction between cyclophosphamide and doxorubicin. Meta-bolism in rat, effect of cyclophosphamide aldoketoreductase system. J. Pharmac. Exp. Ther., 237, 271–74
Urien S., Bree F., Breillout F. (1993): Vinorelbine high affinity binding to human platelets and lymphocytes: distribution in human blood. Cancer Chemother. Pharmacol., 32, 231
Jehl F., Quoix E., Leveque D. (1991): Pharmacokinetic and preliminary metabolic fate of navelbine in humans as determined by high performance liquid chromatography. Cancer Res., 51, 2073
Allen T.M. (1997) Liposomes: opportunities in drug delivery. Drugs 54, 8–14
Amantea M. Gabizon A. (1998): Pharmacokinetics of Caelyx®, Doxil® (Stealth® liposomal doxorubicin) in patients with breast or prostate cancer. Proc. NCI-EORTC (Amsterdam) Abstract No. 653
Gabizon A., Catane R., Uziely B. (1994) Prolonged circulation-time and enhanced accumulation in malignant exsudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 54, 987–992
Northfelt D.W., Francis J.M., Working P., et al. (1996) Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumour localization and safety in patients with AIDS related Kaposi sarcoma. J. Clin. Pharmacol., 36, 55–63.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Linkesch, W., Weger, M., Eder, I. et al. Long-term pharmacokinetics of doxorubicin HCl stealth liposomes in patients after polychemotherapy with vinorelbine, cyclophosphamide and prednisone (CCVP). Eur. J. Drug Metab. Pharmacokinet. 26, 179–184 (2001). https://doi.org/10.1007/BF03190394
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190394