Summary
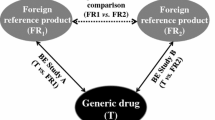
The WHO List of International Comparator Pharmaceutical Products (CPP) For Equivalence Assessment of Interchangeable Multi-Source (Generic) Products will address an important issue in developing new generic drugs because it will identify the ‘correct’ reference product. This list will reduce unnecessary clinical studies in jurisdictions requiring new generics to be compared with brand products sold locally. Eventually, by employing the CPP, there will be a world-wide standard for brand and generic drugs, assuring the same level of quality internationally.
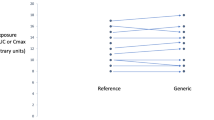
The strategy of a single global reference is meritorious, but there are several hurdles to overcome. Most important is that the same brand may differ in dissolution and/or bioavailability in various jurisdictions, including some drugs with a narrow therapeutic index like phenytoin. Several examples are provided in this manuscript. This issue of regional differences has relevance, not only to the WHO list, but also to the matter of how safety and efficacy was established for that product in the first place. Normally, phase III clinical studies are conducted on a product manufactured in a single site, set to one standard. If the product differs in bioavailability in different jurisdictions, one is left with the question: ‘which product has remained true to the original formulation?’ Alternatively, if safety and efficacy is maintained with all formulations, then one is faced with the question: ‘are the criteria currently employed for bioequivalence unnecessarily restrictive?’
Similar content being viewed by others
References
World Health Organization (1999): Marketing Authorization of Pharmaceutical Products with Special Reference to Multi-Source (Generic) Products: A Manual for a Drug Regulatory Authority. Regulatory Support Series No. 5. Geneva: WHO.
World Health Organization (1999): List of International Comparator Pharmaceutical Products (CPP) For Equivalence Assessment of Interchangeable Multi-Source (Generic) Products. In: report of informal discussions on the selection of comparator pharmaceutical product for equivalent assessment of interchangeable multi-source (generic) products. Geneva: WHO, 8–17.
The European Agency for the Evaluation of Medicinal Products/Human Medicines Evaluation Unit. (1988): Note for Guidance on The Investigation of Bioavailability and Bioequivalence. London.
Food and Drug Administration/Office of Generic Drugs/Division of Bioequivalence. (1992): Statistical Procedures for Bioequivalence Studies using a Standard Two-Treatment Crossover Design.
Food and Drug Administration/Office of Generic Drugs/Division of Bioequivalence. (1993): Oral Extended (Controlled) Release Dosage Forms — In Vivo Bioequivalence and In Vitro Dissolution Testing.
Drugs Directorate, Health Protection Branch. (1992): Conduct and Analysis of Bioavailability and Bioequivalence Studies. Part A: Oral Dosage Formulations used for Systemic Effects. Ottawa, Ontario, Canada.
Therapeutic Products Programme, Health Canada. (1996): Conduct and Analysis of Bioavailability and Bioequivalence Studies. Part B: Oral Modified Release Formulations. Ottawa, Ontario, Canada.
Kumar V. (1991): In-house data supplied by Barr Laboratories. Conjugated Estrogens Pilot Bioequivalency Study.
McGilveray I. (1992): Director, Bureau of Drug Research, Health Canada. Personal communication.
Farinha A., Nobrega S.D., Paulo T. (1997): Evaluation of pharmaceutical quality of phenytoin sodium capsules and tablets from multinational markets. Drug Dev. Ind. Pharmacol., 23, 47–61.
Eichelbaum M., Somogyi A. (1984): Intra- and inter-subject variation in the first-pass elimination of highly cleared drugs during chronic dosing. Studies with deuterated verapamil. Eur. J. Clin. Pharmacol., 26, 47–53.
Hollmann M., Brode E., Hotz D., Kaumeier S., Kehrhahn O.H. (1983): Investigations on the pharmacokinetics of propafenone in man. Drug. Res., 33, 763–770.
Lee J.T., Kroemer H.K., Silberstein D.J. et al. (1990): The role of genetically determined polymorphic drug metabolism in the beta-blockade produced by propafenone. N. Engl. J. Med., 322, 1764–1768.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spino, M., Tsang, Y.C. & Pop, R. Dissolution and in vivo evidence of differences in reference products: impact on development of generic drugs. Eur. J. Drug Metab. Pharmacokinet. 25, 18–24 (2000). https://doi.org/10.1007/BF03190051
Issue Date:
DOI: https://doi.org/10.1007/BF03190051