Summary
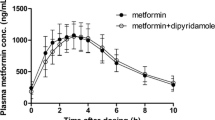
Dipyridamole is a well known anti-aggregating agent characterized by poor water solubility as well as scant and variable bioavailability. Recently, the compound was complexed with β-cyclodextrin forming a molecular encapsulation resulting in better oral absorption and stronger biological activities in animals. In the present study, a randomized double blind cross-over comparison between dipyridamole-β-cyclodextrin complex (dip-β-CD) and dipyridamole was performed in 12 healthy subjects after single (75mg) and multiple oral treatments (75mg TTD). Dip-β-CD showed better bioavailability and less interindividual variability than dipyridamole either after single or multiple doses. In particular, dip-β-CD had a greater AUC and Cmax, and a smaller Tmax even at the steady state. Li addition, 100% of the subjects receiving a single dose of dip-β-CD, as compared to 66.7% of those treated with dipyridamole, had plasma levels superior to 1 μg/ml (which is the supposed anti-aggregating threshold level). In contrast, 0 and 33.03% of the subjects showed plasma levels superior to 2.5 μg/ml (which might cause the appearance of side-effects) on the 7th day of the multiple treatment with dip-β-CD and dipyridamole, respectively. In fact, the subjects presenting higher levels after uncomplexed dipyridamole also complained of headache and/or dizziness on occasion. No adverse side effects were reported for dip-β-CD.
Similar content being viewed by others
References
Nielsen-Kudsk F, Pedersen A.K. (1979): Pharmacokinetics of dipyridamole. Acta Pharmacol. Toxicol., 44, 391–399.
Mellinger T.J., Bohorfoush J.G. (1966): Blood levels of dipyridamole (persantin) in humans. Arch. Int. Pharmacodyn., 163, 471–480.
Manhoy C., Cox M.J., Bjomsson T.D. (1983): Plasma dipyridamole concentrations after two different dosage regimens in patients. J. Clin. Pharmacol., 23, 123–126.
Rajah S.M., Crow M.J., Penny A.F., Ahmad R., Watson D.A. (1977): The effect of dipyridamole on platelet function: correlation with blood levels in man. Br. J. Clin. Pharmacol., 4, 129–133.
Fregnan G.B., Bertè F. (1990): Enhancement of specific biological activity of dipyridamole by complexation with β-cyclodextrin. Pharmacology, 40, 96–102.
Stracciari G.L., Malvisi J., Anfossi P., Fregnan G.B. (1989): Pharmacokinetics of dipyridamole-β-cyclodextrin complex in dogs. Arch. Int. Pharmacodyn., 300, 7–13.
Torn G., Naggi A., Fregnan G.B., Trebbi A. (1990): Dipyridamole-β-cyclodextrin complex: preparation and characterization. Pharmazie., 45, 193–196.
Sedman A.J., Wagner J.G. (1976): CSTRIP, a Fortran IV computer program for obtaining initial polyexponential parameter estimates. J. Pharmacol. Sci., 65, 1006–1010.
Fregnan G.B., Vandoni G., Torn G., Naggi A. (1989): Dipiridamolo-β-ciclodestrina: un nuovo complesso con miglior biodisponibilità. II Giomate italiane di biofarmaceutica e farmacocinetica. Problematiche di rilevante interesse in biofarmaceutica e farmacocinetica. Roma, Italy, 21–22 March. Abstract p. 13.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ricevuti, G., Mazzone, A., Pasotti, D. et al. Pharmacokinetics of dipyridamole-β-cyclodextrin complex in healthy volunteers after single and multiple doses. European Journal of Drug Metabolism and Pharmacokinetics 16, 197–201 (1991). https://doi.org/10.1007/BF03189959
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189959