Summary
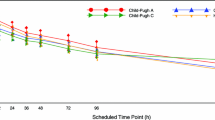
The pharmacokinetics of single 50 mg oral and intravenous doses of milnacipran, a new non tricyclic antidepressant drug, were compared in 11 chronic liver impaired (LI) subjects and in 6 control subjects. Hepatic impairments, classified according to the PUGH scale were moderate (1 grade A), intermediate (6 grade B) and severe (4 grade C). Concentrations of unchanged drug and its conjugated form (its main metabolite) were measured in plasma and urines. In control subjects, milnacipran present high absolute bioavailability (mean value of 90%). Around 50% of the dose are excreted in urines as unchanged, while around 14% are excreted as glucuroconjugate. The remaining is composed of free and conjugated phase I inactive metabolites. Administration of milnacipran in LI subjects results in non significant changes in its pharmacokinetics, although its variability is increased. Unchanged drug exposure is not modified in LI subjects, while plasma levels of the conjugate are slightly decreased compared to the control group. This could either be due to a slight reduction in the conjugation process, or to a change in the distribution of the drug as urine excretion of both unchanged and conjugated forms are not modified compared to the control group. A few LI subjects present supra-bioavailability resulting in higher drug exposure after oral administration than after intravenous infusion. These modifications are not clinically relevant as drug exposure of the parent drug is not modified. As the unchanged drug is the only compound responsible for the activity of milnacipran, no dosage adjustment is needed in patients presenting liver impairment.
Similar content being viewed by others
References
Milnacipran hydrochloride, Dalcipran registered trade mark, Toledomin registered trade mark. Drugs Future. 21(1): 88–89.
Pugh RN, Murray-Lyon IM, Dawson JL et al. (1973). Transection of the oesophagus for bleeding oesophagus varices. Br J Surg. 60: 646–649.
Puozzo C, Duchene P, Bromet M, Filaquier C, Zorza G. (1996). Determination of milnacipran (F2207) and its glucuroconjugate in plasma, blood and urines by high performance liquid chromatography with fluorescence detection. J Chromatogr. To be published.
Rowland M, Tozer T. (1980). Clinical pharmacokinetics concepts and applications. Ed Lea Febinger, Philadelphia.
Puozzo C, Filaquier C, Briley M. (1985). Plasma levels of F2207, a novel antidepressant, after a single oral administration in volunteers. Br J Clin Pharm. 20: 291.
Deprez D, Chassard D, Baille P, Mignot A, Ung HL, Puozzo C. (1998). Which bioequivalence for a racemic drug? Application to midlnacipran. Eur J Drug Metab pharmacokinet. 23 (2): 166–171.
Mc Lean AJ, Morgan DJ. (1991). Clinical pharmacokinetics in patients with liver disease. Clin Pharmacokinet. 21(1): 42–69.
Morgan DJ, Mc Lean AJ. (1995). Clinical pharmacokinetics and pharmacodynamic considerations in patients with liver disease — un update. Clin Pharmacokinet. 29(5): 370–3911.
Puozzo C, Pozet N, Deprez D, Baille P, Ung UL, Zech P. (1998). Pharmacokinetics of milnacipran in renal impairment. Eur J Drug Metab pharmacokinet. 23 (2): 280–286.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Puozzo, C., Albin, H., Vinçon, G. et al. Pharmacokinetics of milnacipran in liver impairment. European Journal of Drug Metabolism and Pharmacokinetics 23, 273–279 (1998). https://doi.org/10.1007/BF03189351
Issue Date:
DOI: https://doi.org/10.1007/BF03189351