Abstract
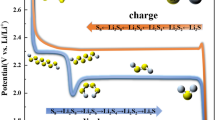
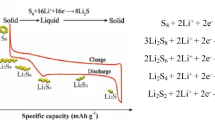
The lithium/sulfur cell is very attractive because of its high theoretical specific capacity and low production cost. The sulfur electrode is prepared from sulfur, with carbon as an electronic conductor and PEO as an ionic conductor. We changed the carbon content of a 50 wt.% sulfur electrode from 10 wt.% to 40 wt.%. The lithium/PEO/sulfur cell showed two plateau potential regions (2.4 V, 2.1 V) and high discharge capacity, i.e., 1484 mAh/g (88% utilization) for optimum composition. The discharge capacity decreased drastically by charge-discharge cycling. The degradation rate as well as the first discharge capacity depended on the composition of the sulfur electrode. The optimum composition of the 50 wt.% sulfur electrode was 30 wt.% carbon and 20% PEO.
Similar content being viewed by others
References
S. S. Lee, K. W. Kim, B. Y. Hur, T. H. Nam, and H. J. Ahn,Met. Mater.-Int. 7, 265 (2001).
J. H. Shin, Y. T. Lim, K. W. Kim, and H. J. Ahn,Met. Mater.-Int. 7, 485 (2001).
R. P. Tischer,The Sulfur Electrode, p. 220, Academic Press (1983).
E. J. Cairns, E. C. Gay, R. K. Steunenberg, H. Shimotake, J. R. Selman, T. L. Wilson, and D. S. Webster,Argonne National Lab. Rep. 7953 (1972).
R. D. Rauh, K. M. Abraham, G. F. Pearson, J. K. Suprenant, and S. B. Brummer,J. Electrochem. Soc. 126, 523 (1979).
E. Peled, A. Gorenshtein, M. Segal, and Y. Sternberg,J. Power Sources 26, 269 (1989).
E. Peled, Y. Sternberg, A. Gorenshtein, and Y. Lavi,J. Electrochem. Soc. 136, 1621 (1989).
Degott,Doctoral Thesis, l’Institutes National Polytechnique de Grenoble, France (1986).
M. Y. Chu,U.S. Patent 5, 532, 077 (1996).
S. C. Han, M. S. Song, H. Lee, H. S. Kim, H. J. Ahn, and J. Y. Lee,J. Electrochem. Soc. 150, A889 (2003).
S. E. Cheon, K. S. Ko, J. H. Cho, S. W. Kim, E. Y. Chin, and H. T. Kim,J. Electrochem. Soc. 150, A796 (2003).
Y. M. Lee, N. S. Choi, J. H. Park, and J. K. Park,J. Power Sources 119–121, 964 (2003).
D. Marmostein, T. H. Yu, K. A. Striebel, F. R. McLarnon, J. Hou, and E. J. Cairns,J. Power Sources 89, 219 (2000).
B. H. Jeon, J. H. Yeon, and I. J. Chung,J. Mater. Proc. Tech. 143–144, 93 (2003).
D. R. Chang, S. H. Lee, S. W. Kim, and H. T. Kim,J. Power Sources 112, 452 (2002).
J. H. Shin, K. W. Kim, H. J. Ahn, and J. H. Ahn,Mater. Sci. Eng. B 95, 148 (2002).
J. H. Shin, Y. T. Lim, K. W. Kim, H. J. Ahn, and J. H. Ahn,J. Power Sources 107, 103 (2002).
J. H. Shin, B. S. Jung, S. S. Jeong, K. W. Kim, H. J. Ahn, K. K. Cho, and J. H. Ahn,Met. Mater.-Int. 10, 177 (2004).
P. T. Cunningham, S. A. Johnson, and E. J. Cairns,J. Electrochem. Soc. 125, 1450 (1972).
I. Barin,Thermochemical Data of Pure Substances, p. 841, VCH, New York (1989).
T. Hemmingsen,Electrochim. Acta 37, 2775 (1992).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is based on a presentation in “The 7th Korea-China Workshop on Advanced Materials” organized by the Korea-China Advanced Materials Cooperation Center and the China-Korea Advanced Materials Cooperation Center, held at Ramada Plaza Jeju Hotel, Jeju Island, Korea on August 24–27, 2003.
Rights and permissions
About this article
Cite this article
Park, CW., Ryu, HS., Kim, KW. et al. Effect of sulfur electrode composition on the electrochemical property of lithium/PEO/sulfur battery. Met. Mater. Int. 10, 375–379 (2004). https://doi.org/10.1007/BF03185988
Issue Date:
DOI: https://doi.org/10.1007/BF03185988