Abstract
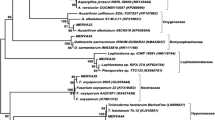
Two strains ofPenicillium, DQ25 and SC10, isolated from marine spongeHaliclona angulata (Bowerbank) andHymeniacidon sp. respectively, were subjected to stationary cultivation under GYP medium for 30 days. The fermentation extracts were undergone bioactivities assays against human pathogens, phytopathogenic fungi and brine shrimp (Artemia salina). Bioassays-guided compounds isolation was performed by Silica gel columns and Sephadex LH-20 chromatography. Spectroscopic methods were used to structures elucidation of the compounds. Results showed the activities of secondary metabolites of strain DQ25 were generally stronger than that of strain SC10. Major bioactive molecules isolated from strain DQ25 were a 1,4-naphthoquinone derivative and an unidentified alkaloid. The two components were not isolated from the extract of strain SC10. ITS sequences revealed that these two species have the greatest similarity withPenicillium vinaceum andPenicillium granulatum respectively.
Similar content being viewed by others
References
Aniszewski T. (2007). Alkaloids-Secrets of Life. Alkaloid Chemistry, Biological Significance, Applications and Ecological Role. Elsevier B.V., Amsterdam, pp. 156–157.
Aoki M., Itezono Y., Shirai H., Nakayama N., Sadai A., Tanaka Y., Tamaguchi A., Shimma N., Yokose K., Seto H. (1991). Structure of a novel phospholipase C inhibitor, Vinaxanthone (Ro 09-1450), produced byPenicillium vinaceum. Tetrahedron Lett., 32: 4737–4740.
Baker P.W., Kennedy J., Dobson A.D., Marchesi J.R. (2009). Phylogenetic diversity and antimicrobial activities of fungi associated withHaliclona simulans isolated from Irish coastal waters. Mar. Biotechnol., 11: 540–547.
Carballo J.L., Hernández-Inda Z.L., Pérez P., García-Grávalos M.D. (2002). A comparison between two brine shrimp assays to detectin vitro cytotoxicity in marine natural products. BMC Biotechnol., 2: 17. http://www. biomedcentral.com/1472-6750/2/17
Ciegler A., Hou C.T. (1970). Isolation of viridicatin fromPenicillium palitans. Arch. Mikrobiol., 73: 261–267.
Ciegler A., Kurtzman C.P. (1970). Penicillic acid production by blue-eye fungi on various agricultural commodities. Appl. Microbiol., 20: 761–764.
Clark R.J., Garson M.J., Hooper J.N. (2001). Antifungal alkyl amino alcohols from the tropical marine spongeHaliclona n. sp. J. Nat.Prod., 64: 1568–1571.
Currie J.N., Thom C. (1915). An oxalic acid producingPenicillium. J. Biol. Chem., 22: 287–293.
Dalsgaard P.W., Blunt J.W., Munro M.H., Frisvad J.C., Christophersen C. (2005). Communesins G and H, new alkaloids from the psychrotolerant fungusPenicillium rivulum. J. Nat. Prod., 68: 258–261.
Ding L., Qin S., Li F., Chi X., Laatsch H. (2008). Isolation, antimicrobial activity, and metabolites of fungusCladosporium sp. associated with red algaPorphyra yezoensis. Curr. Microbiol., 56: 229–235.
Faulkner D.J., Harper M.K., Salomon C.E., Schmidt E.W. (1999). Localisation of bioactive metabolites in marine sponges. In: Hooper J.N.A., Ed., Proceedings of 5th International Sponge Symposium. Mem. Qld. Mus., 44: pp. 167–173.
Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. (2004). Mycotoxins, drugs and other extrolites produced by species inPenicillium subgenusPenicillium. Stud. Mycol., 49: 201–241.
Frisvad J.C., Filtenborg O. (1983). Classification of terverticillate Penicillia based on profiles of mycotoxins and other secondary metabolites. Appl. Environ. Microbiol., 46: 1301–1310.
Jadulco R.C. (2002). Isolation and structure elucidation of bioactive secondary metabolites from marine sponges and sponge-derived fungi. Dissertation of University of Wuerzburg, Germany.
Kapadia G.J., Balasubramanian V., Tokuda H., Konoshima T., Takasaki M., Koyama J., Tagahaya K., Nishino H. (1997). Anti-tumor promoting effects of naphthoquinone derivatives on short term Epstein-Barr early antigen activation assay and in mouse skin carcinogenesis. Cancer Lett.,113: 47–53.
Klich M.A. (1998). Soil fungi of some low-altitude desert cotton fields and ability of their extracts to inhibitAspergillus flavus. Mycopathologia, 142: 97–100.
Li Q, Wang G. (2009). Diversity of fungal isolates from three Hawaiian marine sponges. Microbiol. Res., 164: 233–241.
LimnaMol V.P., Raveendran T.V., Parameswaran P.S. (2009). Antifouling activity exhibited by secondary metabolites of the marine sponge,Haliclona exigua (Kirkpatrick). Int. Biodeterior. Biodegrad., 63: 67–72.
Meazza G., Dayan F.E., Wedge D.E. (2003). Activity of quinones onColletotrichum species. J. Agric. Food Chem., 51: 3824–3828.
Raeder U., Broda P. (1985). Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol., 1: 17–20.
Raper K.B., Thom C. (1949). A Manual of the Penicillia. Williams & Wilkins Co., Baltimore.
Rezanka T., Rezanka P., Sigler K. (2008). A biaryl xanthone derivative having axial chirality fromPenicillium vinaceum. J. Nat. Prod., 71: 820–823.
Schiaparelli S., Albertelli G., Cattaneo-Vietti R. (2003). The epibiotic assembly on the spongeHaliclona dancoi (Topsent, 1901) at Terra Nova Bay (Antarctica, Ross Sea). Polar. Biol., 26: 342–347.
Sera Y, Adachi K, Fujii K, Shizuri Y. (2002). Isolation of haliclonamides: new peptides as antifouling substances from a marine sponge species,Haliclona. Mar. Biotechnol., 4: 441–446.
Sette L.D., Passarini M. R Z., Delarmelina C., Salati F., Duarte M.C.T. (2006). Molecular characterization and antimicrobial activity of endophytic fungi from coffee plants. World J. Microbiol. Biotechnol., 22: 1185–1195.
Shigemori H., Bae M.A., Yazawa K., Sasaki T., Kobayashi J. (1992). Alteramide A, a new tetracyclic alkaloid from a bacteriumAlteromonas sp. associated with the marine spongeHalichondria okadai. J. Org. Chem., 57: 4317–4320.
Solov’eva T.F., Reshetilova T.A., Baskunov B.P., Griforian K.M., Kozlovskiî A.G. (1995). Alkaloids fromPenicillium species fungi isolated from food products. Prikl. Biokhim. Mikrobiol., 31: 545–550.
Unson M.D., Faulkner D.J. (1993). Cyanobacterial symbiont biosynthesis of chlorinated metabolites fromDysidea herbacea (Porifera). Experientia (Basel), 49: 349–353.
Volk C.A., Köck M. (2004). Viscosaline: new 3-alkyl pyridinium alkaloid from the Arctic spongeHaliclona viscose. Org. Biomol. Chem., 13: 1827–1830.
Wischik C.M., Horsley D., Rickard J.E., Harrington C.R. (2005). Napthoquinone derivatives as inhibitors of Tau aggregation for the treatment of Alzheimer’s and related neurodegenerative disorders. US patent: 20050107472 A1.
Zheng L., Yan X., Han X., Chen H., Lin W., Lee F.S., Wang X. (2006). Identification of norharman as the cytotoxic compound produced by the sponge (Hymeniacidon perleve)-associated marine bacteriumPseudoalteromonas piscicida and its apoptotic effect on cancer cells. Biotechnol. Appl. Biochem., 44: 135–142.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wei, R., Li, F., Song, R. et al. Comparison of two marine sponge-associatedPenicillium strains DQ25 and SC10: differences in secondary metabolites and their bioactivities. Ann. Microbiol. 59, 579–585 (2009). https://doi.org/10.1007/BF03175149
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03175149