Abstract
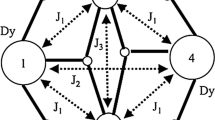
New compounds, [Cu3Ln2(ClCH2COO)12(H2O)8]·2H2O with Ln = Nd3+ (I), Sm3+ (II), Pr3+ (III), built up of pentanuclear clusters were synthesized and studied by means of X-ray analysis and electron paramagnetic resonance (EPR). X-ray data show that all compounds are isostructural and the pentanuclear clusteres may be considered as a linear system with alternating Cu(II) and Ln(III) ions: Cu(2)-L1-Ln-L2-Cu(1)-L2-Ln-L2-Cu(2) with L1 and L2 being bridging fragments and Cu(1) and Cu(2) being structurally nonequivalent copper complexes. EPR studies demonstrate that in the temperature range of 100–293 K the signals due to only one type of the copper complexes are observed in the spectra of I–III. AtT<100 K the spectral temperature dependence is nontrivial. AtT<30 K new signals are detected in the spectra of I and II. The temperature dependence of the EPR spectra is interpreted under the assumption that the parameter of the exchange interaction Cu(2)-Ln considerably exceeds the parameter of the interaction Cu(1)-Ln. EPR spectra are calculated for the fragments of five paramagnetic centers in the frames of the model taking into account the nonequivalence of two copper complexes, short longitudinal and transverse paramagnetic relaxation times of the rare-earth ions at room temperature and the change of the relaxation rates when the temperature decreases. The results of the calculations show that it is possible to obtain information about the interactions in the system on the basis of the analysis of the temperature dependence of the EPR spectra of the central copper complex. The parameter of the isotropic part of the exchange interaction between copper and neodymium ions (for the interaction Cu(2)-Nd) is estimated as about 15 cm−1. A considerable rearrangement of the spin states when the temperature changes is found for all complexes.
Similar content being viewed by others
References
Kahn O.: Molecular Magnetism, p. 380. New York: VCH Publishers 1993; Kahn O. (ed.): Magnetism: A Supramolecular Function (NATO ASI Series, series C, vol. 484). Dordrecht: Kluwer Academic Publishers 1996; Kahn O.: Adv. Inorg. Chem.4. 179–358 (1996); Kahn O.: Struct. Bonding68, 89–167 (1987)
Benelli C., Gatteschi D.: Chem. Rev.102, 2369 (2002); Gatteschi D., Kahn O., Miller J.S., Palacio F. (eds.): Magnetic Molecular Materials (NATO ASI Series, series E, vol. 198). Dordrecht: Kluwer 1991; Miller J.S., Epstein A.J.: Chem. Eng. News1995, 30–42.
Sakamoto M., Manseki K., Okawa H.: Coord. Chem. Rev.219–221, 379–414 (2001); Okawa H., Furutachi H., Fenton D.E.: Coord. Chem. Rev.174, 51–75 (1998)
Avecilla F., Platas-Iglesias C., Rodrigues-Cortinas R., Guillemot G., Bunzli J.-C.G., Brondino C.D., Geraldes C.F.G. de-Blas A., Rodriguez-Blas T.: J. Chem. Soc. Dalton Trans.2002, 4659–4665; Piguet C., Edder C., Rigaut S., Bernardinelli G., Bunzli J.-C.G., Hopfgarther G.: J. Chem. Soc. Dalton Trans.2000, 3999–4006.
Likodimos V., Guskos N., Gamari-Seale H., Koufoudakis A., Wabia M., Typek J., Fuks H.: Phys. Rev. B54, 12342–12352 (1996); Rudra I., Raghu C., Ramasesha S.: Phys. Rev. B65, 224–411 (2002)
XEMP ver. 4.2. Siemens Analytical X-ray Inst. Inc. 1990.
Otwinowski Z., Minor W. in: Methods in Enzymology (Carter C.W., Sweet R.M., eds.), vol. 276, p. 307. London: Academic Press 1996.
Sheldrick G.M.: SHELXL 97. Program for the Refinement of Crystal Structures University of Gottingen, Germany, 1997.
Brown G.M., Chidambaran R.: Acta Crystallogr. B29, 2393 (1973)
Voronkova V.K., Huskowska E., Legendziewicz J., Yablokov Yu.V.: Fiz. Tverd. Tela St. Peterburg39, 2057–2061 (1997)
Salikhov K.M., Galeev R.T., Voronkova V.K., Yablokov Yu.V., Legendziewicz J.: Appl. Magn. Reson.14, 457–472 (1998)
Abragam A., Bleaney B.: Electron Paramagnetic Resonance of Transitions Ions. Oxford: Clarendon Press 1970.
Molin Yu.N., Salikhov K.M., Zamaraev K.I.: Spinovyi Obmen. Novosibirsk: Nauka 1977.
Bencini A., Gatteschi D.: Electron Paramagnetic Resonance of Exchange Coupled Systems. Berlin: Springer 1990.
Yablokov Yu.V., Voronkova V.K., Mosina L.V.: Paramagnitnyi Rezonans Obmennykh Klasterov. Moscow: Nauka 1988.
Voronkova V.K., Yablokov Yu.V., Legendziewicz J., Borzechowska M.: Fiz. Tverd. Tela St. Peterburg41, 2154–2157 (1999)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Voronkova, V.K., Galeev, R.T., Shova, S. et al. Exchange interaction and spin dynamics in pentanuclear clusters, Cu3Ln2(ClCH2COO)12(H2O)8 (Ln = Nd3+, Sm3+, Pr3+). Appl. Magn. Reson. 25, 227–247 (2003). https://doi.org/10.1007/BF03166687
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03166687