Abstract
The Au-SiO2 and Sn-SiO2 complexes have been experimentally calibrated at varying temperature, silica concentration and pH:

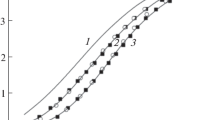
Compared with Au-Cl, Au-HS and Sn-OH complexes, AuH3SiO4 and Sn(H3SiO4)4 complexes can be recognized as the dominant transport forms in Si-bearing solutions under pH and Eh conditions of general interest. The decrease of SiO2 concentration and oxygen fugacity would reverse the direction of dissolution-complexing reactions, resulting in the precipitation of gold and silica, as well as cassiterite and silica. This study illustrates the significance of SiO2-complexation in hydrothermal solutions for gold, tin and other metallizations.
Similar content being viewed by others
References
Chou, I. M., 1989, A low-temperature redox sensor: EOS, v. 70, n. 15, p. 507.
Dadze, T. P., V. I. Sorokhin, and I. Y. Nekrasov, 1982, Solubility of SnO2 in water and in aqueous solutions of HC1, HCl+KCl and HNO3 at 200–400°C and 101.3 MPa: Geochem. Int., v. 19, p. 142–152.
Fan Wenling, Wang Shengyuan, and Wu Jianjun, 1993, Experimental calibration of Au-Si complexation in lowtemperature hydrothermal solutions: Chinese Science Bulletin, v. 38, n. 10, p. 933–935.
Fan Wenling, Wang Shengyuan, and Tian Yifu, 1997, Experimental study on the complexation of SiO2 with the ore-forming element Sb: Acta Mineralogica Sinica, v. 17, n. 4, p. 472–477 (in Chinese with English abstract).
Henley, R.W., 1984, pH calculations of hydrothermal fluids, in fluid-mineral equilibria in hydrothermal systems (Chapter 7) : Reviews in Ecom. Geol., n. 1, p. 90.
Helgeson, H.C., 1969, Thermodynamics of hydrothermal systems at elevated temperatures and pressures: Amer. J.Sci., v. 267, p. 729–804.
Huston, D. L. and R. A. Largers, 1989, Chemical model for the concentration of gold in volcanogenic massive sulfide deposits: Ore Geology Reviews, n. 4, p. 171–200.
Jzckson, K.J. and H.C. Helgeson, 1985, Chemical and thermodynamic constraints on the hydrothermal transport and deposition of tin: I. Calculation of the solubility of cassiterite at high pressures and temperatures : Geochim. et Cosmochim. Acta, v. 49, p. 1–22.
Wood, S.A., D.A. Crerar, and M. P. Boresik, 1987, Solubility of the assemblage pyrite-magnetite-sphaleritegalena-gold-stibnite-bismuthinite-argentite-molybdenite in H2O-NaCl-CO2 solutions from 200°C to 350°C: Econ. Geol., v. 82, p. 1864–1887.
Author information
Authors and Affiliations
Additional information
This research project was granted jointly by the National Natural Science Foundation of China (Grant No. 49373181) and the Open Lab. of Ore Deposit Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences.
Rights and permissions
About this article
Cite this article
Fan, W., Wang, S., Tian, Y. et al. Complexation of Si in hydrothermal systems. Chin. J. of Geochem. 20, 201 (2001). https://doi.org/10.1007/BF03166140
DOI: https://doi.org/10.1007/BF03166140