Abstract
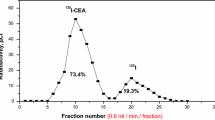
CEA-79 is a murine IgG2a type monoclonal antibody (MoAb) generated using purified CEA from culture supernatants of a human colon cancer cell line, LS174T. The association constant and immunoreactivity of the I-131 labeled CEA-79 ranged from 2.0 to 3.2 ×109 l/mole, and from 54 to 74 %, respectively. The purpose of this study was to evaluate the feasibility of radioimmunoscintigraphy employing MoAb CEA-79 in patients with advanced gastrointestinal carcinomas. Two mgs of MoAb CEA-79 was labeled with 111 MBq (3 mCi) of I-131, and infused intravenously in 6 stomach cancer and 16 colon cancer patients. Out of 6 patients with stomach cancer, immunoscintigraphy was able to detect the tumors in 4 cases. However, immunoscintigraphy found out tumors in all patients with colon cancer. Moreover, 1 patient with stomach cancer and 2 patients with colon cancer showed increased uptake of MoAb in the tumor lesions despite normal serum levels of CEA. We could conclude that this antibody has a potential as a new imaging agent for the diagnosis of gastrointestinal carcinoma.
Similar content being viewed by others
References
Need TE, Neessle W, Ochsner: Carcinoma of the stomach; why are we failing to improve survival.Ann Surg 193: 407–413, 1981
Diel JT, Hermann RE, Cooperman AM, et al: Gastric carcinoma: a ten-year review.Ann Surg 198: 9–12, 1983
Goldenberg DM, DeLand FM, Kim E, et al: Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning.N Engl J Med 298: 1384–1388, 1978
Hine KR, Bradwell AR, Reeder TA, et al: Radio-immunodetection of gastrointestinal neoplasms with antibodies to carcinoembryonic antigen.Cancer Res 44: 2993–2996, 1980
Ishii N, Nakata K, Muro T, et al: Radioimmuno-detection of cancer using antibodies to alpha-fetoprotein and carcinoembryonic antigen.Ann NY Acad Sci 417: 270–276, 1983
Delaloye B, Bischof-Delaloye A, Buchegger F, et al: Detection of colorectal carcinoma by emission-computerized tomography after injection of I-123-labelled Fab or F (ab′)2 fragments from monoclonal anti carcinoembryonic antigen antibodies.J Clin Invest 77: 301–311, 1986
Abdel-Nabi HH, Schwartz AN, Higano CS, et al: Colorectal carcinoma: Detection with indium-111 anticarcinoembryonic-antigen monoclonal antibody ZCE-025.Radiology 164: 617–621, 1987
Carrasquillo JA: Radioimmunoscintigraphy with polyclonal or monoclonal antibodies. InAntibodies in Radio diagnosis and Therapy, Zalutsky MR (ed.), Beca Raton, CRC Press, pp 170–198, 1989
Lee J-H, Chung H-K, Kim S-W: Purification of carcinoembryonic antigen from culture supernatant of human colon cancer cell lines LS174T using monoclonal immunoaffinity chromatography.Korea J Biochem 20: 23–31, 1988
Chung J-K, Hong MK, Jang JJ, et al: Measurement of CEA concentration in various adenocarcinomas byin vitro quantitative autoradiography.J Nucl Med 33: 892, 1992 (abstract)
Lindmo T, Boven E, Cuttitta F, et al: Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess.J Immunol Method 72: 77–85, 1984
Perkins AC, Pimm MV:Immunoscintigraphy: practical aspects and clinical applications, New York, Wiley-Liss, pp 129–162, 1991
Gold P, Freedman SO: Demonstration of tumor specific antigens in human colon carcinoma by immunologic tolerance absorption technique.J Exptl Med 12: 439–462, 1965
Mackay AM, Pater S, Carter S, et al: Role of serial plasma CEA assays in detection of recurrent and metastatic colorectal carcinoma.Br Med J 4: 382–385, 1974
Mach JP, Chetal JK, Lumroso JD, et al: Tumor localization in patients by radiolabelled monoclonal antibodies against colon carcinoma.Cancer Res 43: 5593–5600, 1983
Chung J-K, Lee DS, Lee MC, et al: Establishment of I-131, Tc-99m labeling methods to in-house anti-CEA antibodies and evaluation of the immunological characteristics.Korean J Nucl Med 26: 346–354, 1992
Koong SS, Kim ST, Lee BM, et al: Factors contributing to the localization of the infused monoclonal anti-CEA IgG in human colon carcinoma grafted in the nude mouse.Korean J Int Med 43: 579–589, 1992
Kim EE, DeLand FM, Casper S, et al: Radioimmunodetection of colorectal cancer.Cancer 45: 1243–1247, 1980
Granowska M, Britton KE, Mather SJ, et al: Recent advances in the use of radiolabelled monoclonal antibodies in the management of ovarian cancer. InClinical Use of Antibodies, Baum RP, Cox PH, Hor G, Buraggi GI (eds.), Dordrecht, Kluwer Academic Publishers, pp 91–110, 1991
Author information
Authors and Affiliations
Additional information
This study was supported in part by a grant from the Cancer Research Center of Korea Scientific and Engineering Foundation. (grant number KOSEF SRC-56-CRC-11)
Rights and permissions
About this article
Cite this article
Chung, JK., Choi, C.W., Lee, M.C. et al. Radioimmunoscintigraphy of advanced gastrointestinal carcinomas employing I-131 labeled CEA-79 monoclonal antibody. Ann Nucl Med 7, 65–70 (1993). https://doi.org/10.1007/BF03164570
Issue Date:
DOI: https://doi.org/10.1007/BF03164570