Abstract
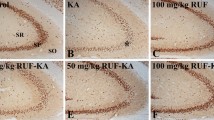
Kainic acid (KA) is a known potent neuroexcitotoxin, although the biochemical mechanism producing its underlying neurotoxic effect is not quite clear. Histopathological examination of gerbil brains 24 h after systemic injection of KA revealed severe neuronal lesions in different regions of the brain,expecially the cerebellar and hippocampal areas. We have detected free radical formation in the brain 1 h after KA administration by using an in vivo spin trapping technique. We have also observed increased lipid peroxidation in the brain after KA-treatment by analyzing thiobarbituric acid reactive substances and conjugated diene formation. Diminished brain specific (Na+, K+)-ATPase activity was also found 2 h after KA injection and persisted to 24 h. It is possible that the free radical reaction is a primary cause of neuronal degeneration after KA administration.
Similar content being viewed by others
References
Ahmad F. F., Cowan D. L., and Sun A. Y. (1987) Detection of free radical formation in various tissues after acute carbon tetrachloride administration in gerbil.Life Sci. 41, 2469–2475.
Beal M. F., Kowall N. W., Ellison D. W., Mazurek M. F., Swartz K. F., and Martin J. B. (1986) Replication of the neurochemical characteristics of Huntington’s disease by quinolinic acid.Nature 321, 168–171.
Berdichevsky E., Riveros N., Sanchez-Ormass S., and Orreg F. (1983) KainateN-methyl aspartate and other excitatory amino acid increases in calcium influx into rat brain cortex cells in vitro.Neurosci. Lett. 36, 75–80.
Biziere K. and Coyle J. T. (1978) Effects of kainic acid on ion distribution and ATP levels of striatal slices incubated in vitro.J. Neurochem. 31, 513–520.
Bloom R. J. and Westerfield W. W. (1971) The thiobarbituric acid reaction in relation to fatty livers.Arch. Biochem. Biophys. 145, 669–675.
Chan P. H., Chu L., Chen S. F., Carlson E. J., and Epstein C. J. (1990) Reduced neurotoxicity in transgenic mice overexpressing human copper-zinc-superoxide dismutase.Stroke 21 (Suppl. 3), 80–82.
Chen G., Griffin M., Poyer J. L., and McCay P. B. (1990) HPLC procedure for the pharmacokinetic study of the spin-trapping agent, α-phenyl-N-tert-butyl nitrone.Free Radical Biol. Med. 8, 93–98.
Cheng Y., Bu Q., Oldfield F. F., Cowan D. L., and Sun A. Y. (1990) The biochemical mechanism of the excitotoxicity of kainic acid.Free Radical Biol. Med. 9(Suppl. 1), 106 (abstract).
Choi D. W. (1985) Glutamate neurotoxicity in cortical cell culture is calcium dependent.Neurosci. Lett. 58, 293–297.
Choi D. W. (1987) Ionic dependence of glutamate neurotoxicity in cortical cell culture.J. Neurosci. 7, 369–379.
Choi D. W. (1988) Calcium-mediated neurotoxicity: Relationship to specific channel types and role in ischemic damage.Trends Neurosci. 11, 465–469.
Choi D. W., Koh J. Y., and Peters S. (1988) Pharmacology of glutamate neurotoxicity in cortical cell culture: Attenuation by NMDA antagonists.J. Neurosci. 8, 185–196.
Coyle J. T. (1988) Neurotoxic actions of kainic acid.J. Neurochem. 41, 1–11.
Coyle J. T. and Schwarcz R. (1976) Lesion of striatal neurons with kainic acid provides a model for Huntington’s chorea.Nature 263, 244–246.
Dumuis A., Sebben M., Haynes L. Pin L.-P., and Bockaert J. (1988) NMDA receptors activate the arachidonate cascade system in striatal neurons.Nature 336, 68–70.
Dykens J. A., Stern A., and Trenkner E. (1987) Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous to reperfusion tissue injury.J. Neurochem. 49, 1222–1228.
Freeman B. A. and Crapo J. D. (1982) Free radicals and tissue injury.Lab. Investig. 47, 412–426.
Gee D. L. and Tappel A. L. (1981) Production of volatile hydrocarbons by isolated hepatocytes: An in vitro model for lipid peroxidation studies.Toxicol. Appl. Pharmacol. 60, 112–120.
Goldberg M. P., Viseskul V., and Choi D. W. (1988) Phencyclidine receptor ligands attenuate cortical neuronal injury afterN-methyl-d-aspartate exposure or hypoxia.J. Pharmacol. Exp. Ther. 245, 1081–1087.
Gutteridge J. M. C. and Halliwell B. (1990) The measurement and mechanisms of lipid peroxidation in biological systems.Trends Biochem. Sci. 15, 129–135.
Koh J. Y. and Choi D. W. (1988) Cultured striatal neurons containing NADPH-diaphorase or acetylcholinesterase are selectively sensitive to injury by NMDA receptor agonists.Brain Res 446, 374–378.
Lazarewicz J. W., Leu V., Sun G. Y., and Sun A. Y. (1983) Arachidonic acid release from K+-evoked depolarization of brain synaptosomes.Neurochem. Int. 5, 471–478.
Lazarewicz J. W., Lehman A., Hagverg H., and Hamberger A. (1986) Effects of kainic acid on brain calcium fluxes studied in vivo and in vitro.J. Neurochem. 46, 494–498.
Lazarewicz J. N., Wroblewski J. T., Palmer M. E., and Costa E. (1988) Activation ofN-methyl-d-aspartate sensitive glutamate receptors stimulates arachidonic acid release in primary cultures of cerebellar granular cells.Neuropharmacology 27, 765–770.
Lazarewicz J. N., Wroblewski J. T., and Costa E. (1990)N-methyl-d-aspartate sensitive glutamate receptors induce, calcium-mediated arachidonic acid release in primary cultures of cerebellar granule cells.J. Neurochem. 55, 1875–1881.
Lothman E. W. and Collins R. L. (1981) Kainic acid induced limbic seizure: Metabolic, behavioral, electroencephalographic and neuropathological correlates.Brain Res. 218, 299–318.
McGeer E. G., McGeer P. L., and Singh K. (1978) Kainate induced degeneration of neostriatal neurons: Dependency upon the corticostriatal tract.Brain Res. 132, 381–383.
Meldrum B. and Garthwaite J. (1990) Excitatory amino acid neurotoxicity and neurodegenerative disease.Trends Pharmacol. Sci. 11, 379–387.
Monaghan D. T. and Cotman C. W. (1982) Distribution of3H-kainate binding sites in rat CNS as determined by autoradiography.Brain Res. 252, 91–100.
Murphy S. N., Thayer S. A., and Miller R. J. (1987) The effects of excitatory amino acids on intracellular calcium in single mouse striatal neurons in vitro.J. Neurosci. 1, 4145–4158.
Murphy T. H., Miyamoto M., Sastre A., Schraar R. L., and Coyle J. T. (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress.Neuron 2, 1547–1558.
Oldfield F. F., Cowan D. L., and Sun A. Y. (1991) The involvement of ethanol in the free radical reaction of 6-hydroxydopamine.Neurochem. Res. 16, 83–87.
Olney J. W., Price M. T., Samson L., and Labruyere J. (1986) The role of specific ions in glutamate neurotoxicity.Neuroscience 65, 65–71.
Olney J., Price M., Salles K. S., Labruyere J., and Frierdich G. (1987) MK-801 powerfully protects againstN-methyl aspartate neurotoxicity.Eur. J. Pharmacol. 141, 357–361.
Recknagel R. O. and Ghoshal A. K. (1966) Quantitative estimation of peroxidative degeneration of rat-liver microsomal and mitochondrial lipids after carbon tetrachloride poisoning.Exp. Mol. Pathol. 5, 413–426.
Reinke L. A., Lai E. K., DuBose C. M., and McCay P. B. (1987) Reactive free radical generationin vivo in heart and liver of ethanol-fed rats: Correlations with radical formationin vitro.Proc. Natl. Acad. Sci. USA 84, 9223–9227.
Retz K. C. and Coyle J. T. (1982) The effects of kainic acid on high energy metabolites in the mouse striatum.J. Neurochem. 38, 196–203.
Rose K., Bruno V. M. G., Oliker R., and Choi O. N. (1990) Nordihydroguaiaretic acid attenuates slow excitatory amino acid-induced neuronal degeneration in cortical cultures.Soc. Neurosci. Abstract 16, 288.
Rothman S. M. (1985) The neurotoxicity of excitatory amino acids is produced by passive chloride influx.J. Neurosci. 5, 1483–1489.
Siesjo B. K. (1984) Cerebral circulation and metabolism.J. Neurosurg. 60, 885–908.
Slater T. F. (1984) Free-radical mechanisms in tissue injury.Biochem. J. 222, 1–15.
Stenram U. (1953) The specificity of the gallocyanin chromalum stain for nucleic acids as studied by the ribonuclease technique.Exp. Cell Res. 4, 383–389.
Sun A. Y. (1972) The effect of lipoxidation on synaptosomal (Na+, K+)-ATPase isolated from the cerebral cortex of squirrel monkey.Biochim. Biophys. Acta 266, 350–360.
Sun A. Y. (1974) The effect of phospholipases on the active uptake of norepinephrine by synaptosomes isolated from the cerebral cortex of guinea pig.J. Neurochem. 22, 551–556.
Sun A. Y. and Sun G. Y. (1976) Functional role of phospholipids of synaptosomal membranes, inFunction and Metabolism of Phospholipids in the Central and Peripheral Systems (Porcellati G., Amaducci L., and Galli C., eds.), pp. 169–197, Plenum, New York.
Sun A. Y., Cheng Y., and Sun G. Y. (1992) Kainic acid-induced excitotoxicity in neurons and glial cells.Prog. Brain Res. (in press).
Unnerstall J. R. and Wamsley J. K. (1983) Autoradiographic localization of high-affinity3H-kainate binding sites in the rat forebrain.Eur. J. Pharmacol. 86, 361–371.
Weiloch T. (1985) Hypoglycemic-induced neuronal damage is prevented byN-methyl-d-aspartate receptor antagonist.Science 230, 681–683.
Wroblewski J. T., Nicoletti F., and Costa E. (1989) Different coupling of excitatory amino acids receptors with Ca2+ channels in primary culture of cerebellar granule cells.Neuropharmacology 241, 919–921.
Author information
Authors and Affiliations
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/BF03160079.
Rights and permissions
About this article
Cite this article
Sun, A.Y., Cheng, Y., Bu, Q. et al. The biochemical mechanisms of the excitotoxicity of Kainic acid. Molecular and Chemical Neuropathology 17, 51–63 (1992). https://doi.org/10.1007/BF03159981
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03159981