Abstract
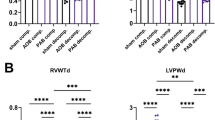
During cardiac maturation, increased exposure of the heart to circulating catecholamines correlates with increased conduction velocity and growth of the heart. We used an in vitro approach to study the underlying mechanisms of adrenergic stimulation induced changes in conduction velocity. By combining functional measurements and molecular techniques, we were able to demonstrate that the increased conduction velocity after β-adrenergic stimulation is probably not caused by changes in intercellular coupling. Instead, RT-PCR experiments and action potential measurements have shown an increased excitability that may well explain the observed increase in conduction velocity. Apart from being relevant to cardiac maturation, our findings are relevant in the context of stem cells and cardiac repair. Preconditioning of stem cell derived cardiomyocytes may help to enhance electrical maturation of de novo generated cardiomyocytes and consequently reduce their proarrhythmogenic potential. (Neth Heart J 2008;16:106-9.)


Similar content being viewed by others
References
Renick SE, Seidler FJ, McCook EC, Slotkin TA. Neuronal control of cardiac and hepatic macromolecule synthesis in the neonatal rat: effects of sympathectomy. Pediatr Res 1997;41:359-63.
Claycomb WC. Biochemical aspects of cardiac muscle differentiation: possible control of deoxyribonucleic acid synthesis and cell differentiation by adrenergic innervation and cyclic adenosine 3’:5’-monophosphate. J Biol Chem 1976,251:6082-89.
Vaidya D, Tamaddon HS, Lo CW, Taffet SM, Delmar M, Morley GE, et al. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res 2001;88:1196-202.
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 2002;383:725-37.
Delorme B, Dahl E, Jarry-Guichard T, Marics I, Briand JP, Willecke K, et al. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev Dyn 1995;204:358-71.
Delorme B, Dahl E, Jarry-Guichard T, Briand JP, Willecke K, Gros D, et al. Expression pattern of connexin gene products at the early developmental stages of the mouse cardiovascular system. Circ Res 1997;81:423-37.
Van Kempen MJ, Vermeulen JL, Moorman AF, Gros D, Paul DL, Lamers WH. Developmental changes of connexin40 and connexin43 mRNA distribution patterns in the rat heart. Cardiovasc Res 1996;32:886-900.
Alcoléa S, Théveniau-Ruissy M, Jarry-Guichard T, Marics I, Tzouanacou E, Chauvin JP, et al. Downregulation of connexin 45 gene products during mouse heart development. Circ Res 1999;84:1365-79.
van Veen TAB, van Rijen HV, Opthof T. Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res 2001;51:217-29.
de Boer TP, van Rijen HVM, van der Heyden MAG, Kok B, Opthof T, Vos MA, et al. Beta- not alpha-adrenergic stimulation enhances conduction velocity in cultures of neonatal cardiomyocytes. Circ J 2007;71:973-81.
van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 2004;109:1048-55.
Thomas SP, Kucera JP, Bircher-Lehmann L, Rudy Y, Saffitz JE, Kléber AG. Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43. Circ Res 2003;92:1209-16.
Cheng Q, Ross RS, Walsh KB. Overexpression of the integrin β1A subunit and the β1A cytoplasmic domain modifies the β-adrenergic regulation of the cardiac L-type Ca2+ current. J Mol Cell Cardiol 2004;36:809-19.
Maki T, Gruver EJ, Davidoff AJ, Izzo N, Toupin D, Colucci W, et al. Regulation of calcium channel expression in neonatal myocytes by catecholamines. J Clin Invest 1996;97:656-63.
Author information
Authors and Affiliations
Corresponding author
Additional information
Department of Medical Physiology, Division of Heart & Lungs, University Medical Center Utrecht, the Netherlands
Interuniversity Cardiology Institute of the Netherlands, Utrecht and Heart Failure Research Center, Academic Medical Center, Amsterdam, the Netherlands
Correspondence to: T.A.B. van Veen Department of Medical Physiology, Division of Heart & Lungs, University Medical Center Utrecht, PO Box 85500, 3508 GA Utrecht, the Netherlands
Rights and permissions
About this article
Cite this article
de Boer, T.P., van Rijen, H.V.M., van der Heyden, M.A.G. et al. Adrenergic regulation of conduction velocity in cultures of immature cardiomyocytes. NHJL 16, 106–109 (2008). https://doi.org/10.1007/BF03086127
Issue Date:
DOI: https://doi.org/10.1007/BF03086127