Abstract
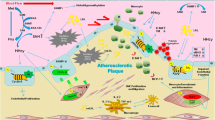
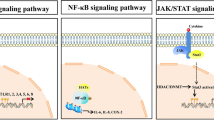
In the past decade, research into cardiovascular diseases, such as atherosclerosis and restenosis, has been focused on the identification of genetic factors that determine disease risk besides clinical risk factors. Many genes in lipid metabolism, vascular homeostasis, haemostasis and inflammation have been found to be related to coronary artery disease1 and the multifactorial nature of the disease suggests a role for many other, yet uninvestigated genes. Previous research from our department has demonstrated the importance of genetics in restenosis after a percutaneous coronary intervention (PCI). Polymorphisms in several inflammatory genes, such as TNFα, eotaxin, CD14, GM-CSF, IL-10, caspase-1, but also noninflammatory genes, such as LPL, stromelysin-1 and the β adrenergic receptor have been found to be associated with the risk of restenosis.2-5 It has become clear, however, that part of the gene-environmental interactions relevant for complex diseases is regulated by epigenetic mechanisms such as histone acetylation and DNA methylation.

Similar content being viewed by others
References
Nordlie MA, Wold LE, Kloner RA. Genetic contributors toward increased risk for ischemic heart disease. J Mol Cell Cardiol 2005;39: 667-79.
Monraats PS, Pires NM, Schepers A, Agema WR, Boesten LS, de Vries MR, et al. Tumor necrosis factor-alpha plays an important role in restenosis development. FASEB J 2005;19: 1998-2004.
Monraats PS, Pires NM, Agema WR, Zwinderman AH, Schepers A, de Maat MP, et al. Genetic inflammatory factors predict restenosis after percutaneous coronary interventions. Circulation 2005;112: 2417-25.
Monraats PS, Rana JS, Nierman MC, Pires NM, Zwinderman AH, Kastelein JJ, et al. Lipoprotein lipase gene polymorphisms and the risk of target vessel revascularization after percutaneous coronary intervention. J Am Coll Cardiol 2005;46: 1093-100.
Monraats PS, de Vries F, de Jong LW, Pons D, Sewgobind VD, Zwinderman AH, et al. Inflammation and apoptosis genes and the risk of restenosis after percutaneous coronary intervention. Pharmacogenet Genomics 2006;16: 747-54.
Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 2005;102: 10604-9.
Jenuwein T, Allis CD. Translating the histone code. Science 2001;293: 1074-80.
Wu C. Chromatin remodeling and the control of gene expression. J Biol Chem 1997;272: 28171-4.
Grunstein M. Histone acetylation in chromatin structure and transcription. Nature 1997;389: 349-52.
Santini V, Gozzini A, Ferrari G. Histone deacetylase inhibitors: molecular and biological activity as a premise to clinical application. Curr Drug Metab 2007;8: 383-93.
Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, et al. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem 2003;278: 2758-66.
Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 2002;9: 625-36.
Ashburner BP, Westerheide SD, Baldwin AS, Jr. The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol 2001;21: 7065-77.
de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 2003;370: 737-49.
Sambucetti LC, Fischer DD, Zabludoff S, Kwon PO, Chamberlin H, Trogani N, et al. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem 1999;274: 34940-7.
Okamoto H, Fujioka Y, Takahashi A, Takahashi T, Taniguchi T, Ishikawa Y, et al. Trichostatin A, an inhibitor of histone deacetylase, inhibits smooth muscle cell proliferation via induction of p21(WAF1). J Atheroscler Thromb 2006;13: 183-91.
Mnjoyan ZH, Dutta R, Zhang D, Teng BB, Fujise K. Paradoxical upregulation of tumor suppressor protein p53 in serum-stimulated vascular smooth muscle cells: a novel negative-feedback regulatory mechanism. Circulation 2003;108: 464-471.
Kusama H, Kikuchi S, Tazawa S, Katsuno K, Baba Y, Zhai YL, et al. Tranilast inhibits the proliferation of human coronary smooth muscle cells through the activation of p21waf1. Atherosclerosis 1999;143: 307-13.
Lee B, Kim CH, Moon SK. Honokiol causes the p21WAF1-mediated G(1)-phase arrest of the cell cycle through inducing p38 mitogen activated protein kinase in vascular smooth muscle cells. FEBS Lett 2006;580: 5177-84.
Kato S, Ueda S, Yamaguchi M, Miyamoto T, Fujii T, Izumaru S, et al. Overexpression of p21Waf1 induces apoptosis in immortalized human vascular smooth muscle cells. J Atheroscler Thromb 2003;10: 239-45.
Patel VI, Daniel S, Longo CR, Shrikhande GV, Scali ST, Czismadia E, et al. A20, a modulator of smooth muscle cell proliferation and apoptosis, prevents and induces regression of neointimal hyperplasia. FASEB J 2006;20: 1418-30.
Yan C, Wang H, Toh Y, Boyd DD. Repression of 92-kDa type IV collagenase expression by MTA1 is mediated through direct interactions with the promoter via a mechanism, which is both dependent on and independent of histone deacetylation. J Biol Chem 2003;278: 2309-16.
Jayaraman G, Srinivas R, Duggan C, Ferreira E, Swaminathan S, Somasundaram K, et al. p300/cAMP-responsive element-binding protein interactions with ets-1 and ets-2 in the transcriptional activation of the human stromelysin promoter. J Biol Chem 1999;274: 17342-52.
Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, et al. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 2004;18: 1897-9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Department of Cardiology, Leiden University Medical Centre, Leiden, and the Interuniversity Cardiology Institute of the Netherlands, Utrecht, the Netherlands
J.W. Jukema Department of Cardiology, Leiden University Medical Centre, PO Box 9600, 2300 RC Leiden, the Netherlands
Rights and permissions
About this article
Cite this article
Pons, D., Jukema, J.W. Epigenetic histone acetylation modifiers in vascular remodelling – new targets for therapy in cardiovascular disease. NHJL 16, 30–32 (2008). https://doi.org/10.1007/BF03086114
Issue Date:
DOI: https://doi.org/10.1007/BF03086114