Abstract
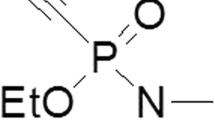
In this work, two oximes for the treatment of tabun-inhibited acetylcholinesterase (AChE; EC 3.1.1.7), K074 (1,4-bis(4-hydroxyiminomethyl-pyridinium) butane dibromide) and K075 ((E)-1,4-bis(4-hydroxyiminomethylpyridinium)but-2-en dibromide), were testedin vitro as reactivators of AChE. Comparison was made with currently used AChE reactivators (pralidoxime, HI-6, methoxime and obidoxime). Human brain homogenate was taken as an appropriate source of the cholinesterases. As resulted, oxime K074 appears to be the most potent reactivator of tabun-inhibited AChE, with reactivation potency comparable to that of obidoxime. A second AChE reactivator, K075, does not attain as great a reactivation potency as K074, although its maximal reactivation (17%) was achieved at relevant concentrations for humans.
Similar content being viewed by others
References
Bajgar J (2004) Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment.Adv. Clin. Chem. 38, 151–216.
Bajgar J and J Herink (1995) Continual monitoring of the rat bloode cholinesterase activityin vivo following administration of different inhibitors.Toxicol. Lett. 78(Suppl. 1), 18.
Bartosova L K Kuca, G Kunesova and D Jun (2006) The acute toxicity of acetylcholinesterase reactivators in mice in relation to their structure.Neurotoxicity Res. 9, 291–296.
Cabal J and J Bajgar (1999) Tabun - reappearance 50 years later.Chem. Listy 93, 27–31 (in Czech).
Cabal J, K Kuca and J Kassa (2004) Specification of the structure of oximes able to reactivate tabun inhibited acetylcholinesterase.Bas. Clin. Pharmacol. Toxicol. 95, 81–86.
De Jong LP, MA Verhagen, JP Langenberg, I Hagedorn and M Loffler (1989) The bispyridinium-dioxime HLo-7. A potent reactivator for acetylcholinesterase inhibited by the stereoisomers of tabun and soman.Biochem. Pharmacol. 38, 633–640.
Eto M (1976)Organophosphorus Pesticides: Organic and Biological Chemistry (CRC Press: Cleveland).
Kassa J (2002) Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents.J. Toxicol. Clin. Toxicol. 40, 803–816.
Kassa J (2006) The influence of oxime and anticholinergic drug selection on the potency of antidotal treatment to counteract acute toxic effects of tabun in mice.Neurotoxicity Res. 9, 59–62.
Kassa J and J Cabal (1999a) A comparison of the efficacy of a new asymmetric bispyridinium oxime BI-6 with presently used oximes and H oximes against sarin byin vitro andin vivo methods.Hum. Exp. Toxicol. 18, 560–565.
Kassa J and J Cabal (1999b) A comparison of the efficacy of acetylcholine reactivators against cyclohexyl methylphos-phonofluoridate (GF agent) byin vitro andin vivo methods.Pharmacol. Toxicol. 84, 41–46.
Kassa J and J Cabal (1999c) A comparison of the efficacy of a new asymmetric bispyridinium oxime BI-6 with currently available oximes and H oximes against soman byin vitro andin vivo methods.Toxicology 132, 111–118.
Kassa J and G Kunesova (2006) The influence of antidotal treatment of low-level tabun exposure on cognitive functions in rats using a water maze.Neurotoxicity Res. 9, 39–45.
Kim TH, K Kuca, D Jun and YS Jung (2005) Design and synthesis of new bis-pyridinium oximes as cyclosarin-inhibited acetylcholinesterase reactivators.Bioorg. Med. Chem. Lett. 15, 2914–2917.
Koplovitz I, R Menton, C Matthews, M Shutz, C Nalls and S Kelly (1995) Dose-response effects of atropine and HI-6 treatment of organophosphorus poisoning in guinea pigs.Drug Chem. Toxicol. 18, 119–136.
Kuca K and J Kassa (2003) A comparison of the ability of a new bispyridinium oxime-1-(4-hydroxyiminomethylpyri-dinium)-4-(4-carbamoylpyridinium)butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase byin vitro methods.J. Enzyme Inhib. Med. Chem. 18, 529–535.
Kuca K, J Patocka and J Cabal (2003) Reactivation of organo-phosphate inhibited acetylcholinesterase activity by α,ω-bis- (4-hydroxyiminomethylpyridinium)alkanesin vitro.J. Appl. Biomed. 1, 207–211.
Kuca K, J Patocka, J Cabal and D Jun (2004) Reactivation of organophosphate-inhibited acetylcholinesterase by quaternary pyridinium aldoximes.Neurotoxicity Res. 6, 565–570.
Kuca K, J Cabal, K Musilek, D Jun and J Bajgar (2005) Effective bisquaternary reactivators of tabun-inhibited AChE.J. Appl. Toxicol. 25, 491–495.
Kuca K, D Jun and K Musilek (2006) Structural requirements of acetylcholinesterase reactivators.Mini Rev. Med. Chem. 6, 269–277.
Maekawa K (1995) The sarin poisoning incident in Tokyo subway. Oral presentation: The Fifth International Symposium on Protection Against CBWA, Stockholm, June 11-16. Marrs TC (1993) Organophosphate poisoning.Pharmacol. Ther. 58, 51–66.
Musilek K, K Kuca, D Jun, V Dohnal and M Dolezal (2005) Synthesis of the novel series of bispyridinium compounds bearing xylene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase.J. Enzyme Inhib. Med. Chem. 20, 409–415.
Musilek K, K Kuca, D Jun, V Dohnal and M Dolezal (2006) Synthesis of the novel series of bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activty against chlorpyrifos-inhibited acetylcholinesterase.Biorg. Med. Chem. Lett. 16, 622–627.
Palecek J, V Cirkva, S Relich, L Slavetinska, K Kuca and D Jun (2005) Assignment of organophosphorus-inhibited acetylcholinesterase reactivators structures on the basis of nuclear magnetic resonance spectra.Zprav. Voj. Farm. 15, 14–25 (In Czech).
Pang YP, TM Kollmeyer, F Hong, JC Lee, PI Hammond, SP Haugabouk and S Brimijoin. (2003) Rational design of alkylene-linked bis-pyridiniumaldoximes as improved ace-tylcholinesterase reactivators.Chem. Biol. 10, 491–502.
Patocka J, J Bielavsky and F Ornst (1970) Reactivating effect of β,É-bis-(4-pyridinealdoxime)-2-trans-butene dibromide on isopropyl-methylphosphonylated acetylcholinesterase.FEBSLett. 10, 182–184.
Picha J, K Kuca, M Kivala, M Kohout, J Cabal and F Liska (2005) New group of monoquaternary reactivators of the acetylcholinesterase inhibited by nerve agents.J. Enzyme Inhib. Med. Chem. 20, 233–237.
Poziomek EJ, BE Hackley and GM Steinberg (1958) Pyridinium aldoximes.J. Org.Chem. 23, 714–717.
Taylor P(1996) Anticholinergicagents, In:The Pharmacological Basis of Therapeutics (Hardman JG and LE Limbird, Eds.) (McGraw Hill:New York, NY), pp 161–176.
Worek F, T Kirchner, M Bäcker and L Szinicz (1996) Reactivation by various oximes of human erythrocyte acetylcholinesterase inhibited by different organophosphorus compounds.Arch. Toxicol. 70, 497–503.
Worek FC Diepold and P Eyer (1999) Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics.Arch. Toxicol. 73, 7–14.
Worek F, H Thiermann, L Szinicz and P Eyer (2004) Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes.Biochem Pharmacol. 68, 2237–2248.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuca, K., Cabal, J., Jun, D. et al. In vitro reactivation potency of acetylcholinesterase reactivators — K074 and K075 — to reactivate tabun-inhibited human brain cholinesterases. neurotox res 11, 101–106 (2007). https://doi.org/10.1007/BF03033389
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03033389