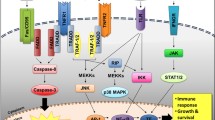
Abstract
The paradoxical occurrence of peripheral cytopenias despite a normo/hypercellular marrow in myelodysplastic syndromes (MDS) has been attributed to excessive intramedullary hematopoietic cell apoptosis. It has also been postulated that abrogation of programmed cell death (PCD) may underlie MDS transformation to acute myeloid leukemia (AML). Despite overwhelming evidence for a role of aberrant apoptosis in myelodysplasia, the molecular mechanisms responsible for such changes have not been elucidated. This paper summarizes current evidence implicating a role for altered PCD in MDS and outlines potential cellular mechanisms whereby hematopoietic progenitor cell apoptosis may be dysregulated.
Similar content being viewed by others
References
Mufti GJ, Galton DA. Myelodysplastic syndromes: natural history and features of prognostic importance.Clin Haematol. 1986;15:953–971.
Yoshida Y. Hypothesis: apoptosis may be the mechanism responsible for the premature intramedullary cell death in the myelodys-plastic syndrome.Leukemia. 1993;7:144–146.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics.Br J Cancer. 1972;26:239–257.
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages.J Immunol. 1992;148:2207–2216.
Martin SJ, Cotter TG. Ultraviolet B irradiation of human leukemia HL-60 cells in vitro induces apoptosis.Int J Radiat Biol. 1991;59:1001–1016.
Hickman JA. Apoptosis induced by anticancer drugs.Cancer Metast Rev. 1992;11:121–139.
Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis.J Immunol. 1988;141:2629–2634.
Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family.Cell. 1993;75:1169–1178.
Williams GT, Smith CA, Spooncer E, Dexter TM, Taylor DR. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis.Nature. 1990;343:76–79.
Thornberry NA, Lazebnik Y. Caspases: enemies within.Science. 1998;281:1312–1316.
Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex.Cell. 1996;85:817–827.
Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death.Cell. 1996;85:803–815.
Li P, Nijhawan D, Budihardjo I, et al. Cytochome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade.Cell. 1997;91:479–489.
Pan G, Humke EW, Dixit VM. Activation of caspases triggered by cytochrome c in vitro.FEBS Lett. 1998;426:151–154.
Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta.Nature. 2000;403:98–103.
Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates and functions during apoptosis.Ann Rev Biochem. 1999;68:383–424.
Nagata S, Golstein P. The Fas death factor.Science. 1995;267:1449–1456.
Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen.J Biol Chem. 1993;268:10932–10937.
Banner DW, D’Arcy A, Janes W, et al. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation.Cell. 1993;73:431–435.
Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD,a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis.Cell. 1995;81:505–512.
Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation.Cell. 1995;81:495–504.
Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-depend-ent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex.Immunity. 1996;4:387–396.
Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways.Cell. 1996;84:299–308.
Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-depend-ent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor.EMBO J. 1995;14:5579–5588.
Binder C, Schulz M, Hiddemann W, Oellerich M. Capsase activation and induction of inducible nitric oxide-synthase during TNF alpha-triggered apoptosis.Anticancer Res. 1999;19:1715–1720.
Green DR, Reed JC. Mitochondria and apoptosis.Science. 1998;281:1309–1312.
Liu X, Kim CN, Yang J, Jemmerson, R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c.Cell. 1996;86:147–157.
Zou H, Henzel WJ, Liu XS, Lutschg A, Wang XD. Apaf-1, a human protein homologous to C-elegans CED-4, participates in cytochrome c-dependent activation of caspase-3.Cell. 1997;90:405–413.
Koseki T, Inohara N, Chen, S, Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases.Proc Natl Acad Sci U S A. 1998;95:5156–5160.
Koseki T, Inohara N, Chen, S, et al. CIPER, a novel NF kappaB-activating protein containing a caspase-recruitment domain with homology to Herpesvirus-2 protein E10.J Biol Chem. 1999;274:9955–9961.
Johnson DE. Programmed cell death regulation: basic mechanisms and therapeutic opportunities.Leukemia. 2000;14:1340–1344.
Marchetti P, Castedo M, Susin SA, et al. Mitochondrial permeability transition is a central coordinating event of apoptosis.J Exp Med. 1996;184:1155–1160.
Skulachev VP. Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide-producing mitochondria and cell.FEBS Lett. 1996;397:7–10.
Desagher S, Osen-Sand A, Nichols A, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis.J Cell Biol. 1999;44:891–901.
Shimizu S, Tsujimoto Y. Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity.Proc Natl Acad Sci U S A. 2000;97:577–582.
Mancini M, Nicholson DW, Roy S, et al. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling.J Cell Biol. 1998;140:1485–1495.
Susin SA, Lorenzo HK, Zamzami N, et al. Mitochondrial release of caspase-2 and -9 during the apoptotic process.J Exp Med. 1999;189:381–394.
Susin SA, Lorenzo HK, Zamzami,N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor.Nature. 1999;397:441–446.
Lorenzo HK, Susin SA, Penninger JM, Kroemer G. Apoptosis-inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death.Cell Death Diff. 1999;6:516–524.
Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death.Blood. 1996;88:386–401.
Reed JC. Bcl-2 and the regulation of programmed cell death.J Cell Biol. 1994;124:1–5.
Muchmore SW, Sattler M, Liang H, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death.Nature. 1996;381;335–341.
Minn AJ, Velez P, Schendel SL, et al. Bcl-x(L) forms an ion channel in synthetic lipid membranes.Nature. 1997;385:353–357.
Schendel SL, Xie ZH, Montal MO, Matsuyama S, Montal M, Reed JC. Channel formation by antiapoptotic protein Bcl-2.Proc Natl Acad Sci U S A. 1997;94:5113–5118.
Jurgensmeier JM, Xie Z, Devereaux Q, Ellerby L, Bredesden D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria.Proc Natl Acad Sci U S A. 1998;95:4997–5002.
Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death.Cell. 1993;74:609–619.
Kharbanda S, Pandey P, Schofield L, et al. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis.Proc Natl Acad Sci U S A. 1997;94:6939–6942.
Reed JC. Double identity for proteins of the Bcl-2 family.Nature. 1997;387:773–776.
Hu Y, Benedict MA, Wu D, Inohara N, Nunez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation.Proc Natl Acad Sci U S A. 1998;95:4386–4391.
Naumovski L, Cleary ML. The p53-binding protein 53BP2 also interacts with Bc12 and impedes cell cycle progression at G2/M.Mol Cell Biol. 1996;16:3884–3892.
Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria.Cell. 1996;87:629–638.
Gottlieb RA, Nordberg J, Skowronski E, Babior BM. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification.Proc Natl Acad Sci U S A. 1996;93:654–658.
Matsuyama S, Xu Q, Velours J, Reed JC. The mitochondrial F0F1-ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells.Mol Cell. 1998;1:327–336.
Sheikh MS, Fornace AJ Jr. Death and decoy receptors and p53-mediated apoptosis.Leukemia. 2000;14:1509–1513.
MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL.J Biol Chem. 1997;272:25417–25420.
Marsters SA, Sheridan JP, Pitti RM, et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain.Curr Biol. 1997;7:1003–1006.
Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL.FEBS Lett. 1997;416:329–334.
Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors.Science. 1997;277:818–821.
Emery JG, McDonnell P, Burke MB, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL.J Biol Chem. 1998;273:14363–14367.
Pitti RM, Marsters SA, Lawrence DA, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer.Nature. 1998;396:699–703.
Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas.Science. 1995;268:411–415.
Condorelli G, Vigliotta G, Cafieri A, et al. PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis.Oncogene. 1999;18:4409–4415.
Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP.Nature. 1997;388:190–195.
Srinivasula SM, Ahmad M, Ottilie S, et al. FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis.J Biol Chem. 1997;272:18542–18545.
Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains.Science. 1999;283:543–546.
Duckett CS, Nava VE, Gedrich RW, et al. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors.EMBO J. 1996;15:2685–2694.
Liston P, Roy N, Tamai K, et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes.Nature. 1996:379;349–353.
Uren AG, Pakusch M, Hawkins CJ, Puls KL, Vaux DL. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors.Proc Natl Acad Sci U S A. 1996;93:4974–4978.
Seol DW, Billiar TR. A caspase-9 variant missing the catalytic siteis an endogenous inhibitor of apoptosis.J Biol Chem. 1999;274:2072–2076.
Srinivasula SM, Ahmad M, Guo Y, et al. Identification of an endogenous dominant-negative short isoform of caspase-9 that can regulate apoptosis.Cancer Res. 1999;59:999–1002.
Clark DM, Lampert IA. Apoptosis is a common histopathological finding in myelodysplasia: the correlate of ineffective haemato-poiesis.Leuk Lymphoma. 1990;2:415–418.
Hatfill SJ, Fester ED, Steytler JG. Apoptotic megakaryocyte dysplasia in the myelodysplastic syndromes.Hem Pathol. 1992;6:87–93.
Bogdanovic AD, Trpinac DP, Jankovic GM, Bumbasirevic VZ, Obradovic M, Colovic MD. Incidence and role of apoptosis in myelodysplastic syndrome: morphological and ultrastructural assessment.Leukemia. 1997;11;656–659.
Greenberg PL, Ginzton N, Rajapaksa R, Tong CR, Han JH. Apoptosis in myelodysplastic syndrome (MDS) [abstract].Blood. 1994;84:159a.
Raza A, Gezer S, Mundle S, et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes.Blood. 1995;86:268–2676.
Rajapaksa R, Ginzton N, Rott LS, Greenberg PL. Altered onco-protein expression and apoptosis in myelodysplastic syndrome marrow cells.Blood. 1996;88:4275–4287.
Kawabata H, Anzai N, Ueda Y, et al. High levels of Ca(2+)-inde-pendent endonuclease activity capable of producing nucleosomal-size DNA fragmentation in non-adherent marrow mononuclear cells from patients with myelodysplastic syndromes and acute myelogenous leukemia.Leukemia. 1996;10:67–73.
Hellstrom-Lindberg E, Kanter-Lewensohn L, Ost A. Morphological changes and apoptosis in bone marrow from patients with myelodysplastic syndromes treated with granulocyte-CSF and ery-thropoietin.Leuk Res. 1997;21:415–425.
Ali A, Mundle SD, Ragasa D, et al. Sequential activation of cas-pase-1 and caspase-3-like proteases during apoptosis in myelodys-plastic syndromes.J Hematother Stem Cell Res. 1999;8:343–856.
Tsoplou P, Kouraklis-Symeonidis A, Thanopoulou E, Zikos P, Orphanos V, Zoumbos NC. Apoptosis in patients with myelodys-plastic syndromes: differential involvement of marrow cells in “good” versus “poor” prognosis patients and correlation with apoptosis-related genes.Leukemia. 1999;13:1554–1563.
Kurotaki H, Tsushima Y, Nagai K, Yagihashi S. Apoptosis, bcl-2 expression and p53 accumulation in myelodysplastic syndrome, myelodysplastic-syndrome-derived acute myelogenous leukemia and de novo acute myelogenous leukemia.Acta Haematologica. 2000;102:115–123.
Anzai N, Kawabata H, Hirama T, et al. Marked apoptosis of human myelomonocytic cell line P39. Significance of cellular differentiation.Leukemia. 1994;8:446–453.
Darzynkiewicz Z, Bedner E, Traganos F, Murakami T. Critical aspects in the analysis of apoptosis and necrosis.Human Cell. 1998;11:3–12.
Lepelley P, Campergue L, Grardel N, Preudhomme C, Cosson A, Fenaux P. Is apoptosis a massive process in myelodysplastic syndromes?Br J Haematol. 1996;95:368–371.
Shetty V, Hussaini S, Broady-Robinson L, et al. Intramedullary apoptosis of hematopoietic cells in myelodysplastic syndrome patients can be massive: apoptotic cells recovered from high-density fraction of bone marrow aspirates.Blood. 2000;96:1388–1392.
Parker JE, Fishlock KL, Czepulkowski B, Mijovic A, Pagliuca A, Mufti GJ. “Low risk” myelodysplastic syndrome (MDS) is associated with excessive apoptosis and an increased ratio of proversus anti-apoptotic Bcl-2 related proteins.Br J Haematol. 1998;103:1075–1082.
Greenberg PL. Apoptosis and its role in the myelodysplastic syndromes: implications for disease natural history and treatment.Leuk Res. 1998;22:1123–1136.
Parker JE, Mufti GJ, Rasool F, Mijovic A, Devereux S, Pagliuca A. The role of apoptosis, proliferation and the Bcl-2 related proteins in the myelodysplastic syndromes (MDS) and acute myeloid leukaemia secondary to MDS (MDS-AML).Blood. 2000;96:3932–3938.
Clark BR, Gallagher JT, Dexter TM. Cell adhesion in the stromal regulation of haemopoiesis.Baillieres Clin Haem. 1992;5:619–652.
Marsh JC, Chang J, Testa NG, Hows JM, Dexter TM. In vitro assessment of marrow “stem cell” and stromal cell function in aplastic anaemia.Br J Haematol. 1991;78:258–267.
Tuzuner N, Cox C, Rowe JM, Watrous D, Bennett JM. Hypocellular myelodysplastic syndromes (MDS): new proposals.Br J Haematol. 1995;91:612–617.
Coutinho LH, Geary CG, Chang J, Harrison C, Testa NG. Functional studies of bone marrow haemopoietic and stromal cells in the myelodysplastic syndrome (MDS).Br J Haematol. 1990;75:16–25.
Silverman LR, Zinzar S, Holland JF. Biological consequences of stromal abnormalities in the myelodysplastic syndrome (MDS): its influence on the hematopoietic dysregulation [abstract].Leuk Res. 1997;21:S20.
Aizawa S, Nakano M, Iwase O, et al. Bone marrow stroma from refractory anemia of myelodysplastic syndrome is defective in its ability to support normal CD34-positive cell proliferation and differentiation in vitro.Leuk Res. 1999;23:239–246.
Aizawa S, Hiramoto M, Hoshi H, Toyama K, Shima D, Handa H. Establishment of stromal cell line from an MDS RA patient which induced an apoptotic change in hematopoietic and leukemic cells in vitro.Exp Hematol. 2000;28:148–155.
List AF, Glinsmann-Gibson B, Spier C, Taetle R. In vitro and in vivo response to cyclosporin-A in myelodysplastic syndromes: identification of a hypocellular subset responsive to immune suppression [abstract].Blood. 1992;80:28a.
Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity.Proc Natl Acad Sci U S A. 1995;92:8115–8119.
Peddie CM, Wolf R, McLellan LI, Collins AR, Bowen DT. Oxidative DNA damage in CD34+ myelodysplastic cells is associated with intracellular redox changes and elevated plasma tumour necrosis factor-a concentration.Br J Haematol. 1997;99:625–631.
Verhoef GE, De Schouwer P, Ceuppens JL, Van Damme J, Goossens W, Boogaerts MA. Measurement of serum cytokine levels in patients with myelodysplastic syndromes.Leukemia. 1992;6:1268–1272.
Kitagawa M, Saito I, Kuwata T, et al. Overexpression of tumor necrosis factor (TNF)-a and interferon (IFN)-γ by bone marrow cells from patients with myelodysplastic syndromes.Leukemia. 1997;11:2049–2054.
Gersuk GM, Beckham C, Loken MR, et al. A role for tumour necrosis factor-a, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome.Br J Haematol. 1998;103:176–188.
Mundle SD, Reza S, Ali A, et al. Correlation of tumor necrosis factor alpha (TNF alpha) with high Caspase 3-like activity in myelodysplastic syndromes.Cancer Lett. 1999;140:201–207.
Deeg HJ, Beckham C, Loken MR, et al. Negative regulators of hemopoiesis and stroma function in patients with myelodysplastic syndrome.Leuk Lymphoma. 2000;37:405–414.
Musto P, Matera R, Minervini MM, et al. Low serum levels of tumor necrosis factor and interleukin-1 beta in myelodysplastic syndromes responsive to recombinant erythropoietin.Haemato-logica. 1994;79:265–268.
Stasi R, Brunetti M, Bussa S, et al. Serum levels of tumour necrosis factor-alpha predict response to recombinant human erythropoietin in patients with myelodysplastic syndrome.Clin Lab Haematol. 1997;19:197–201.
Shetty V, Mundle S, Alvi S, et al. Measurement of apoptosis, proliferation and three cytokines in 46 patients with myelodysplastic syndromes.Leuk Res. 1996;20:891–900.
Reza S, Dar S, Andric T, et al. Biologic characteristics of 164 patients with myelodysplastic syndromes.Leuk Lymphoma. 1999;33:281–287.
Raza A, Qawi H, Lisak L, et al. Patients with myelodysplastic syndromes benefit from palliative therapy with amifostine, pentoxi-fylline, and ciprofloxacin with or without dexamethasone.Blood. 2000;95:1580–1587.
Maurer AB, Ganser A, Buhl R, et al. Restoration of impaired cytokine secretion from monocytes of patients with myelodysplastic syndromes after in vivo treatment with GM-CSF or IL-3.Leukemia. 1993;7:1728–1733.
Bowen D, Yancik S, Bennett L, Culligan D, Resser K. Serum stem cell factor concentration in patients with myelodysplastic syndromes.Br J Haematol. 1993;85:63–66.
Visani G, Zauli G, Tosi P, et al. Impairment of GM-CSF production in myelodysplastic syndromes.Br J Haematol. 1993;84:227–231.
Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis.Cell. 1991;66:233–243.
Miyawaki T, Uehara T, Nibu R, et al. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood.J Immunol. 1992;149:3753–3758.
Leithauser F, Dhein J, Mechtersheimer G, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells.Lab Invest. 1993;69:415–429.
Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils.J Exp Med. 1996;184:429–440.
Moller P, Henne C, Leithauser F, et al. Coregulation of the APO-1 antigen with intercellular adhesion molecule-1 (CD54) in tonsillar B cells and coordinate expression in follicular center B cells and in follicle center and mediastinal B-cell lymphomas.Blood. 1993;81:2067–2075.
Maciejewski J, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro.Blood. 1995;85:3183–3196.
Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection.Nature. 1995;377:630–632.
Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege.Science. 1995;270:1189–1192.
Kitagawa M, Yamaguchi S, Takahashi M, et al. Localization of Fas and Fas ligand in bone marrow cells demonstrating myelodysplasia.Leukemia. 1998;12:486–492.
Bouscary D, De Vos J, Guesnu M, et al. Fas/Apo-1 (CD95) expression and apoptosis in patients with myelodysplastic syndromes.Leukemia. 1997;11:839–845.
Lepelley P, Grardel N, Erny O, et al. Fas/APO-1 (CD95) expression in myelodysplastic syndromes.Leuk Lymphoma. 1998;30:307–312.
Mundle SD, Mativi BY, Bagai K, et al. Spontaneous down-regulation of Fas-associated phosphatase-1 may contribute to excessive apoptosis in myelodysplastic marrows.Int J Hematol. 1999;70:83–90.
O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand.J Exper Med. 1996;184:1075–1082.
Hahne M, Rimoldi D, Schroter M, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape.Science. 1996;274:1363–1366.
Gupta P, Niehans GA, LeRoy SC, et al. Fas ligand expression in the bone marrow in myelodysplastic syndromes correlates with FAB subtype and anemia, and predicts survival.Leukemia. 1999;13:44–53.
Mundle SD, Venugopal P, Cartlidge JD, et al. Indication of an involvement of interleukin-1 beta converting enzyme-like protease in intramedullary apoptotic cell death in the bone marrow of patients with myelodysplastic syndromes.Blood. 1996;88:2640–2647.
Campos L, Sabido O, Viallet A, Piselli S, Guyotat D. Implication of ICE and CPP32 in the growth defects of committed progenitors from myelodysplastic syndromes [abstract].Blood. 1997;90:521a.
Bouscary D, Chen YL, Guesnu M, et al. Activity of the caspase-3/CPP32 enzyme is increased in “early stage” myelodysplastic syndromes with excessive apoptosis, but caspase inhibition does not enhance colony formation in vitro.Exp Hematol. 2000;28:784–791.
Boudard D, Sordet O, Vasselon C, et al. Expression and activity of caspases 1 and 3 in myelodysplastic syndromes.Leukemia. 2000;14:2045–2051.
Delia D, Aiello A, Soligo D, et al. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells.Blood. 1992;79;1291–1298.
Ter-Harmsel B, Smedts F, Kuijpers J, Jeunink M, Trimbos B, Ramaekers F. BCL-2 immunoreactivity increases with severity of CIN: a study of normal cervical epithelia, CIN, and cervical carcinoma.J Pathol. 1996;179:26–30.
Krajewska M, Krajewski S, Epstein JI, et al. Immunohistochemical analysis of bcl-2, bax, bcl-x, and mcl-1 expression in prostate cancers.Am J Pathol. 1996;148:1567–1576.
Leiter U, Schmid RM, Kaskel P, Peter RU, Krahn G. Antiapoptotic bcl-2 and bcl-xL in advanced malignant melanoma.Arch Dermatol Research. 2000;292:225–232.
Aguilar-Santelises M, Rottenberg ME, Lewin N, Mellstedt H, Jondal M. Bcl-2, Bax and p53 expression in B-CLL in relation to in vitro survival and clinical progression.Int J Cancer. 1996;69:114–119.
Pepper C, Hoy T, Bentley DP. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance.Br J Cancer. 1997;76:935–938.
Campos L, Rouault JP, Sabido O, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy.Blood. 1993;81:3091–3096.
Bradbury DA, Zhu YM, Russell NH. Bcl-2 expression in acute myeloblastic leukaemia: relationship with autonomous growth and CD34 antigen expression.Leuk Lymphoma. 1997;24:221–228.
Davis RE, Greenberg PL. Bcl-2 expression by myeloid precursors in myelodysplastic syndromes: relation to disease progression.Leuk Res. 1998;22:767–767.
Lepelley P, Soenen V, Preudhomme C, Merlat A, Cosson A, Fenaux P. bcl-2 expression in myelodysplastic syndromes and its correlation with hematological features, p53 mutations and prognosis.Leukemia. 1995;9:726–730.
Sherr CJ. Cancer cell cycles.Science. 1996;274:1672–1677.
Bincoletto C, Saad ST, Soares da Silva E, Queiroz ML. Autonomous proliferation and bcl-2 expression involving haematopoietic cells in patients with myelodysplastic syndrome.Br J Cancer. 1998;78:621–624.
Raza A, Alvi S, Borok RZ, et al. Excessive proliferation matched by excessive apoptosis in myelodysplastic syndromes: the cause-effect relationship.Leuk Lymphoma. 1997;27:111–118.
Jonveaux P, Fenaux P, Quiquandon I, et al. Mutations in the p53 gene in myelodysplastic syndromes.Oncogene. 1991;6:2243–2247.
Sugimoto K, Hirano N, Toyoshima H, et al. Mutations of the p53 gene in myelodysplastic syndrome (MDS) and MDS-derived leukemia.Blood. 1993;81:3022–3026.
Wattel E, Preudhomme C, Hecquet B, et al. p53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies.Blood. 1994;84:3148–3157.
Uchida T, Kinoshita T, Nagai H, et al. Hypermethylation of the p15INK4B gene in myelodysplastic syndromes.Blood. 1997;90:1403–1409.
Quesnel B, Guillerm G, Vereecque R, et al. Methylation of the p15(INK4b) gene in myelodysplastic syndromes is frequent and acquired during disease progression.Blood. 1998;91:2985–2990.
Karsdorf A, Dresch C, Metral J, Najean Y. Prognostic value of the combined suicide level of granulocyte progenitors and the labelling index of precursors in preleukemic states and oligoblastic leukemias.Leuk Res. 1983;7:279–286.
Montecucco C, Riccardi A, Traversi E, et al. Flow cytometric DNA content in myelodysplastic syndromes.Cytometry. 1983;4:238–243.
Peters SW, Clark RE, Hoy TG, Jacobs A. DNA content and cell cycle analysis of bone marrow cells in myelodysplastic syndromes (MDS).Br J Haematol. 1986;62:239–245.
Raza A, Alvi S, Broady-Robinson L, et al. Cell cycle kinetic studies in 68 patients with myelodysplastic syndromes following intravenous iodo- and/or bromodeoxyuridine.Exp Hematol. 1997;25:530–535.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Parker, J.E., Mufti, G.J. The Role of Apoptosis in the Pathogenesis of the Myelodysplastic Syndromes. Int J Hematol 73, 416–428 (2001). https://doi.org/10.1007/BF02994003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02994003