Abstract
Background
Scintigraphic image analysis of99mTc-mertiatide (Mag-3, mercaptoacetyltriglycine) clearance provides the determination of the blood flow, the tubular transit time and the excretion as well from both kidneys. Radiopharmaceutical routine recommends a radiochemical purity control before administration of the product to a patient. The main objective of this study is to develop a Mag-3 labeling procedure that fits better than the previous one in our daily routine production of radiopharmaceuticals.
Methods
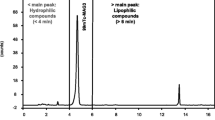
Increasing proportions of99mTc-Mag-3 were measured during the heating and cooling steps of the Mag-3 labeling procedure. HPLC analysis was used to confirm the results of a rapid radiochemical quality control assay on standard ITLC-SG paper. Results: The reconstitution time takes 20-25 minutes from the harvest of pertechnetate to a ready-for-use calibrated patient syringe. The HPLC profile of99mTc-Mag-3 including its minor impurities remains unchanged for 24-48 hours after reconstitution.
Conclusions
The application of a programmable Peltier-directed device for heating/cooling provides a better control of the temperature course. The procedure proposed fully meets the labeling criteria recommended by the supplier and can be performed with a minimum of attention within a time-span that we formerly needed for solely the radiochemical purity control assay. Moreover,99mTc-Mag-3 prepared in this way seems to be considerably more stable than mentioned in the manufacturer’s instructions.
Similar content being viewed by others
References
Technescan® MAG3 package insert (DRN 4334, Dutch version, RVG 16527, 9 Nov 1995), Tyco Healthcare, Mallinckrodt Medical B.V., Petten, the Netherlands.
Nosco DL, Wolfangel RG, Bushman MJ, Grummon GD, Marmion ME, Pipes DW. Technetium-99m-Mag-3: labeling conditions and quality control.J Nucl Med Technol 1993; 21: 69–74.
Van Hemert FJ, Schimmel KJM, Van Eck-Smit BLF. A rapid and stable ITLC procedure for the determination of the radiochemical purity of99mTc-tetrofosmin.Nucl Med Commun 2001; 22; 641–644.
Chen F, Decristoforo C, Rohrbacher B, Riccabona G. A simple two-strip method to determine the radiochemical purity of technetium-99m mercaptoacetyltriglycine.Eur J Nucl Med 1993; 20: 334–338.
Millar AM, Wilkinson AG, McAteer E, Best JJK.99mTc-Mag-3:in vitro stability andin vivo behaviour at different times after preparation.Nucl Med Commun 1990; 11: 405–412.
Brandau W, Bubeck B, Eisenhut M, Taylor DM. Techne-tium-99m labelled renal function and imaging agents: III. Synthesis of99mTc-Mag-3 and biodistribution of by-products.Appl Radiat hot 1988; 39: 121–129.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Hemert, F.J., van Lenthe, H., Schimmel, K.J.M. et al. Preparation, radiochemical purity control and stability of99mTc-mertiatide (Mag-3). Ann Nucl Med 19, 345–349 (2005). https://doi.org/10.1007/BF02984631
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02984631