Abstract
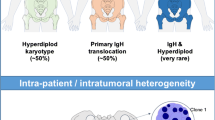
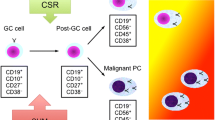
Multiple myeloma (MM) is an incurable malignant neoplasm affecting terminally differentiated B-cells. It derives from post-germinal center B-cells and develops as a result of multistep tumorigenic events, because approximately one third of all MM cases have a history of preceding monoclonal gammopathy of undetermined significance (MGUS) or smoldering myeloma. MM terminates in the formation of extramedullary invasion or in secondary plasma cell leukemia. To account for this clinical experience, investigators have found that intrinsic chromosomal instability followed by complex chromosomal translocations/deletions plays a crucial role in the development from MGUS to MM. Representative aberrations include chromosomal rearrangements involving 14q32 loci and deletion at the long arm of chromosome 13. Contributing to the progression of MM itself are genomic instability and altered methylation of the specific gene promoters. The former results in activation of specific oncogenes such asRAS andFGFR3 or in inactivation ofp53, and the latter results in inactivation of tumor suppressor genes, includingp16. An accurate understanding of each of these molecular events should help clarify the development of specific molecular targeting therapies based on the differences in dysfunctional signaling pathways found in the cells of all MM patients.
Similar content being viewed by others
References
Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions.Nat Rev Cancer. 2002;2:175–187.
Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance.New Engl J Med. 2002;346:564–569.
Drach J, Angerler J, Schuster J, et al. Interphase fluorescence in situ hybridization identifies chromosomal abnormalities in plasma cells from patients with monoclonal gammopathy of undetermined significance.Blood. 1995;86:3915–3921.
Drach J, Schuster J, Nowotny H, et al. Multiple myeloma: high incidence of chromosomal aneuploidy as detected by interphase fluorescence in situ hybridization.Cancer Res. 1995;55:3854–3859.
Nishida K, Tamura A, Nakazawa N, et al. The Ig heavy chain gene is frequently involved in chromosomal translocations in multiple myeloma and plasma cell leukemia as detected by in situ hybridization.Blood. 1997;90:526–534.
Rao PH, Cigudosa JC, Ning Y, et al. Multicolor spectral karyotyping identifies new recurring breakpoints and translocations in multiple myeloma.Blood. 1998;92:1743–1748.
Tai YT, Teoh G, Lin B, et al. Ku86 variant expression and function in multiple myeloma cells is associated with increased sensitivity to DNA damage.J Immunol. 2000;165:6347–6355.
Kato M, Iida S, Komatsu H, Ueda R. Lack of Ku80 alteration in multiple myeloma.Jpn J Cancer Res. 2002;93:359–362.
Difilippantonio MJ, Zhu J, Chen HT, et al. DNA repair protein Ku80 suppresses chromosomal aberration and malignant transformation.Nature. 2000;404:510–514.
Gao Y, Ferguson DO, Xie W, et al. Interplay of p53 and DNArepair protein XRCC4 in tumorigenesis, genomic stability and development.Nature. 2000;404:897–900.
Nigg EA. Centrosome aberrations: cause or consequence of cancer progression?Nat Rev Cancer. 2002;2:815–825.
Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma.Oncogene. 2001;20:5611–5622.
Iida S, Hanamura I, Suzuki T, et al. A novel human multiple myeloma-derived cell line, NCU-MM-1, carrying t(2;11)(p11;q23) and t(8;22)(q24;q11) chromosomal translocations with overexpression of c-Myc protein.Int J Hematol. 2000;72:85–91.
Seto M, Yamamoto K, Iida S, et al. Gene rearrangement and overexpression of PRAD1 in lymphoid malignancy with t(11;14) (q13;q32) translocation.Oncogene. 1992;7:1401–1406.
Chesi M, Bergsagel PL, Brents LA, Smith CM, Gerhard DS, Kuehl WM. Dysregulation of cyclin D1 by translocation into an IgH gamma switch region in two multiple myeloma cell lines.Blood. 1996;88:674–681.
Janssen JWG, Vaandrager J-W, Heuser T, et al. Concurrent activation of a novel putative transforming gene, myeov, and cyclin D1 in a subset of multiple myeloma cell lines with t(11;14)(q13;q32).Blood. 2000;15:2691–2698.
Shaughnessy J Jr, Gabrea A, Qi Y, et al. Cyclin D3 at 6p21 is dysregulated by recurrent chromosomal translocations to immunoglobulin loci in multiple myeloma.Blood. 2001;98:217–223.
Iida S, Rao PH, Butler M, et al. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma.Nat Genet. 1997; 17:226–230.
Yoshida S, Nakazawa N, Iida S, et al. Detection of MUM1/IRF4- IgH fusion in multiple myeloma.Leukemia. 1999;13:1812–1816.
Chesi M, Nardini E, Brents LA, et al. Frequent translocation t(4; 14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3.Nat Genet. 1997;16:260–264.
Chesi M, Nardini E, Lim RSC, Smith KD, Kuehl WM, Bergsagel PL. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts.Blood. 1998;92:3025–3034.
Chesi M, Bergsagel PL, Shonukan OO, et al. Frequent dysregulation of the c-maf proto-oncogene at 16q23 by translocation to an Ig locus in multiple myeloma.Blood. 1998;91:4457–4463.
Avet-Loiseau H, Gerson F, Magrangeas F, Minvielle S, Harousseau J-H, Bataille R. Rearrangements of the c-myc oncogene are present in 15% of primary human multiple myeloma tumors.Blood. 2001;98:3082–3086.
Shou Y, Martelli ML, Gabrea A, et al. Diverse karyotypic abnormalities of the c-myc locus associated with c-myc dysregulation and tumor progression in multiple myeloma.Proc Natl Acad Sci U S A. 2000;97:228–233.
Hanamura I, Iida S, Akano Y, et al. Ectopic expression of MAFB gene in human myeloma cells carrying (14;20)(q32;q11) chromosomal translocations.Jpn J Cancer Res. 2001;92:638–644.
Hatzivassiliou G, Miller I, Takizawa J, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy.Immunity. 2001;14:277–289.
Keats JJ, Reiman T, Maxwell CA, et al. In multiple myeloma, t(4; 14)(p16;q32) is an adverse prognostic factor irrespective of FGFR3 expression.Blood. 2003;101:1520–1529.
Plowright EE, Li Z, Bergsagel PL, et al. Ectopic expression of fibroblast growth factor receptor 3 promotes myeloma cell proliferation and prevents apoptosis.Blood. 2000;95:992–998.
Pollett JB, Trudel S, Stern D, Li ZH, Stewart AK. Overexpression of the myeloma-associated oncogene fibroblast growth factor receptor 3 confers dexamethasone resistance.Blood. 2002;100:3819–38211.
Avet-Loiseau H, Facon T, Daviet A, et al. 14q32 translocations and monosomy 13 observed in monoclonal gammopathy of undetermined significance delineate a multistep process for the oncogenesis of multiple myeloma.Cancer Res. 1999;59:4546–4550.
Fonseca R, Bailey RJ, Ahmann GJ, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance.Blood. 2002;15:1417–1424.
Avet-Loiseau H, Facon T, Grosbois B, et al. Oncogenesis of multiple myeloma: 14q32 and 13q chromosomal abnormalities are not randomly distributed, but correlate with natural history, immunological features, and clinical presentation.Blood. 2002;99:2185–21911.
Moreau P, Facon T, Leleu X, et al. Recurrent 14q32 translocations determine the prognosis of multiple myeloma, especially in patients receiving intensive chemotherapy.Blood. 2002;100:1579–15833.
Fonseca R, Blood EA, Oken MM, et al. Myeloma and the t(11;14) (q13;q32): evidence for a biologically defined unique subset of patients.Blood. 2002;99:3735–3741.
Avet-Loiseau H, Li JY, Morineau N, et al. Monosomy 13 is associated with the transition of monoclonal gammopathy of undetermined significance to multiple myeloma: Intergroupe Francophone du Myélome.Blood. 1999;94:2583–2589.
Shaughnessy J Jr, Tian E, Sawyer J, et al. High incidence of chromosome 13 deletion in multiple myeloma detected by multiprobe interphase FISH.Blood. 2000;96:1505–1511.
Desikan R, Barlogie B, Sawyer J, et al. Results of high-dose therapy for 1000 patients with multiple myeloma: durable complete remissions and superior survival in the absence of chromosome 13 abnormalities.Blood. 2000;95:4008–4010.
Zojer N, Konigsberg R, Ackermann J, et al. Deletion of 13q14 remains an independent adverse prognostic variable in multiple myeloma despite its frequent detection by interphase fluorescence in situ hybridization.Blood. 2000;95:1925–1930.
Facon T, Avet-Loiseau H, Guillerm G, et al. Chromosome 13 abnormalities identified by FISH analysis and serum 2-microglobulin produce a powerful myeloma staging system for patients receiving high-dose therapy.Blood. 2001;97:1566–1571.
Neri A, Murphy JP, Cro L, et al. Ras oncogene mutation in multiple myeloma.J Exp Med. 1989;170:1715–1725.
Corradini P, Ladetto M, Voena C, et al. Mutational activation of Nand K-ras oncogenes in plasma cell dyscrasias.Blood. 1993;81:2708–27133.
Kalakonda N, Rothwell DG, Scarffe JH, Norton JD. Detection of N-Ras codon 61 mutations in subpopulations of tumor cells in multiple myeloma at presentation.Blood. 2001;98:1555–1560.
Bezieau S, Devilder M-C, vet-Loiseau H, et al. High incidence of N and K-ras activating mutations in multiple myeloma and primary plasma cell leukemia at diagnosis.Hum Mutat. 2001;18:212–224.
Liu P, Leong T, Quam L, et al. Activating mutations of N- and Kras in multiple myeloma show different clinical associations: analysis of the Eastern Cooperative Oncology Group phase III trial.Blood. 1996;88:2699–2706.
Seremetis S, Inghirami G, Ferrero D, et al.Transformation and plasmacytoid differentiation of EBV-infected human B lymphocytes by ras oncogenes.Science. 1989;243:660–663.
Balladeau D, Jelinek DF, Shah N, Tucker W, Ness BV. Introduction of an activated N-ras oncogene alters the growth characteristics of the interleukin 6-dependent myeloma cell line ANBL6.Cancer Res. 1995;55:3640–3646.
Chesi M, Brents LA, Ely SA, et al. Activated fibroblast growth factor receptor 3 is an oncogene that contributes to tumor progression in multiple myeloma.Blood. 2001;97:729–736.
Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells.Immunity. 1999;10:105–115.
Hsu J-H, Shi Y, Krajewski S, et al. The AKT kinase is activated in multiple myeloma tumor cells.Blood. 2001;98:2853–2855.
Mitsiades N, Mitsiades CS, Poulaki V, et al. Biologic sequelae of nuclear factor-B blockade in multiple myeloma: therapeutic applications.Blood. 2002;99:4079–4086.
Pettersson M, Jernberg-Wiklund H, Larsson L-G, et al. Expression of the bcl-2 gene in human multiple myeloma cell lines and normal plasma cells.Blood. 1992;79:495–502.
Schwarze MMK, Hawley RG. Prevention of myeloma cell apoptosis by ectopic bcl-2 expression or interleukin 6-mediated upregulation of bcl-xL.Cancer Res. 1995;55:2262–2265.
Zhang B, Gojo I, Fenton RG. Myeloid cell factor-1 is a critical survival factor for multiple myeloma.Blood. 2002;99:1885–1893.
Ng MHL, Chung YF, Lo KW, Wickham NWR, Lee JCK, Huang DP. Frequent hypermethylation of p16 and p15 genes in multiple myeloma.Blood. 1997;89:2500–2506.
Uchida T, Kinoshita T, Ohno T, Ohashi H, Nagai H, Saito H. Hypermethylation of p16INK4A gene promoter during the progression of plasma cell dyscrasia.Leukemia. 2001;15:157–165.
Gonzalez M, Mateos MV, Garcia-Sanz R, et al. De novo methylation of tumor suppressor gene p16/INK4a is a frequent finding in multiple myeloma patients at diagnosis.Leukemia. 1999;14:183–1877.
Kramer A, Schultheis B, Bergmann J, et al. Alterations of cyclin D1/pRb/p16INK4A pathway in multiple myeloma.Leukemia. 2002; 16:1844–1851.
Guillerm G, Gyan E, Wolowiec D, et al. p16INK4a and p15INK4b gene methylations in plasma cells from monoclonal gammopathy of undetermined significance.Blood. 2001;98:244–246.
Neri A, Baldini L, Trecca D, Cro L, Polli E, Maiolo AT. p53 mutations in multiple myeloma are associated with advanced forms of malignancy.Blood. 1993;81:128–135.
Drach J, Ackermann J, Fritz E, et al. Presence of a p53 gene deletion in patients with multiple myeloma predicts for shorter survival after conventional-dose chemotherapy.Blood. 1998;92:802–809.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Iida, S., Ueda, R. Multistep Tumorigenesis of Multiple Myeloma: Its Molecular Delineation. Int J Hematol 77, 207–212 (2003). https://doi.org/10.1007/BF02983776
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02983776