Abstract
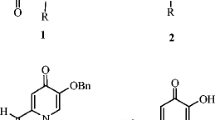
This paper for the first time reports the isolation of 5 compounds fromPhellinus linteus. A sphingolipid (1) and two tyrosinase inhibitory compounds (2, 3) along with two carboxylic acids (4, 5), were isolated from the fruiting body ofPhellinus linteus (Berk & Curt) Aoshima. The structure of compound 1 was identified as 1-O-β-D-glucopyranosyl-(2S, 3R, 4E, 8E)-2-[(2R)-2-hydroxyhexadecanoylamino]-9-methyl-4,8-octadecadiene-1,3-diol, known as cerebroside B, based on spectroscopic methods such as 1D and 2D NMR as well as by acid hydrolysis. Compounds2~5 were identified as protocatechualdehyde (2), 5-hydroxymethyl-2-furaldehyde (HMF) (3), succinic acid (4), and fumaric acid (5) based on the spectroscopic evidence. Compounds2 and3 inhibited the oxidation of L-tyrosine catalyzed by mushroom tyrosinase with an IC50 of 0.40 and 90.8 μg/mL, respectively. The inhibitory kinetics, which were analyzed by the Lineweaver-Burk plots, were found to be competitive and noncompetitive inhibitors with a Ki of 1.1 μM and 1.4 mM, respectively.
Similar content being viewed by others
References
Agrawal, P. K., NMR spectroscopy in the structural elucidation of oligosaccharides.Phytochemistry, 31, 3307–3330 (1992).
Andersen, S. O., Biochemistry of insect cuticle.Annu. Rev. Entomol., 24, 29–61 (1979).
Atkinson, P. W., Brown, W. V., and Gilby, A. R., Phenolic compounds from insect cuticle: Identification of some lipid antioxidants.Insect Biochem., 3, 309–315 (1973).
Cho, J. H., Cho, S. D., Hu, H., Kim, S. H., Lee, S. K., Lee, Y. S., and Kang, K. S., The roles of ERK 1/2 and p38 MAP kinases in the preventive mechanisms of mushroomPhellinus linteus against the inhibition of gap junctional intercellular communication by hydrogen peroxide.Carcinogenesis, 23, 1163–1169 (2002).
Conrad, J. S., Dawso, S. R., Hubbard, E. R., Meyers, T. E., and Strothkamp, K. G., Inhibitor binding to the binuclear active site of tyrosinase: temperature, pH, and solvent deuterium isotope effects.Biochemistry, 33, 5739–5744 (1994).
Dai, Y. C. and Xu, M. Q., Studies on the medicinal polypore,Phellinus baumii, and its kin,P. linteus.Mycotaxon., 67, 191–200 (1998).
Dillehay, D. L., Webb, S. J., Schmelz, E. M., and Merrill, A. H. Jr., Dietary sphingomyelin inhibits 1,2-dimethylhydrazineinduced colon cancer in CF1 mice.J. Nutr., 124, 615–620 (1994).
Gaver, R. C. and Sweeley, C. C., Chemistry and metabolism of spingolipids, 3-oxyderivatives ofN-acetylsphingosine andN- acetyldihydrosphingosine.J. Am. Chem. Soc., 88, 3643–3647 (1966).
Han, S. B., Lee, C. W., Jeon, Y. J., Hong, N. D., Yoo, I. D., Yang, K. H., and Kim, H. M., The inhibitory effect of polysaccharides isolated fromPhellinus linteus on tumor growth and metastasis.Immunopharmacol., 41, 157–164 (1999).
Hwang, S. Y., Hwang, B. Y., Choi, W. H., Jung, H. J., Huh, J. D., Lee, K. S., and Ro, J. S., Quantitative determination of 5- hydroxymethyl-2-furaldehyde in the Rehmanniae radix preparata samples at various processing stages.Kor. J. Pharmacogn., 32, 116–120 (2001).
Ichikawa, K., Kimoshita, T., and Sankawa, U., The screening of Chinese crude drugs for Ca2+ antagonist activity: Identification of active principles from the aerial part of Pogostemon cablin and the Fruits ofPrunus mume.Chem. Pharm. Bull., 37, 345–348 (1989).
Ikekawa, T., Nakanish, M., Uehara, N., Chihara, G., and Fukuoka, F., Antitumor action of some Basidiomycetes, especiallyPhellinus linteus.Gann, 59, 155–157 (1968).
Janzowski, C., Glaab, V., Samimi, E., Schlatter, J., and Eisenbrand, G., 5-Hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione.Food Chem. Toxicol., 38, 801–809 (2000).
Jung, J. H., Lee, C. O., Kim, Y. C., and Kang, S. S., New bioactive cerebrosides fromArisaema amurense.J. Nat. Prod., 59, 319–322 (1996).
Kang, S. S., Kim, J. S., Son, K. H., Kim, H. P., and Chang, H. W., Cyclooxygenase-2 inhibitory cerebroside from Phytolaccae radix.Chem. Pharm. Bull., 49, 321–324 (2001).
Kang, S. S., Kim, J. S., Xu, Y. N., and Kim, Y. H., Isolation of a new cerebroside from the root bark ofAralia elata.J. Nat. Prod., 62, 1059–1060 (1999).
Kikuzaki, H., Kawai, Y., and Nakatani, N., 1,1-Diphenyl-2- picrylhydrazyl radical-scavenging active compounds from greater cardamom (Amomum subulatum Roxb.).J. Nutr. Sci. Vitaminol., 47, 167–171 (2001).
Kim, D. H., Choi, H. J., and Bae, E. A., Effect of artificially culturedPhellinus linteus on harmful intestinal bacterial enzymes and rat intestinal oc-glucosidases.J. Food Hyg. Safety, 13, 20–23 (1998).
Kim, H. M., Han, S. B., Oh, G. T., Kim, Y. H., Hong, D. H., Hong, M. D., and Yoo, I. D., Stimulation of humoral and cell mediated immunity by polysaccharide from mushroomPhellinus linteus.Int. J. Immunopharmacol., 18, 295–303 (1996).
Kim, S. Y., Choi, Y. H., Kim, J. W., Kim, Y.C., and Lee, H. S., New antihepatotoxic cerebroside fromLycium chinense Fruits.J. Nat. Prod., 60, 274–276 (1997).]
Kim, Y. M., Yun, J., Lee, C. K., Lee, H. H., Min, K. R., and Kim, Y. S., Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechamism of action.J. Biol. Chem., 277, 16340–16344 (2002).
Kobayashi, T., Shimizugawa, T., Osakabe, T., Watanabe, S., and Okuyama, H., A long-term feeding of sphingolipids affected the levels of plasma cholesterol and hepatic triacylglycerol but not tissue phospholipids and sphingolipids.Nutr. Res., 17, 111–114 (1997).
Kubo, I. and Kinst-Hori, I., Tyrosinase inhibitors from cumin.J. Agric. Food Chem., 46, 5338–5341 (1998a).
Kubo, I. and Kinst-Hori, I., Tyrosinase inhibitors from anise oil.J. Agric. Food Chem., 46, 1268–1271 (1998b).
Liu, G. T., Ahang, T. M., Wang, B. E., and Wang, Y. W., Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes.Biochem. Pharmacol., 43, 147–152 (1992).
Liu, Y., Xie, P., and Wang, B., Evaluation of radixSalvae miltiorrhizae and its preparation.Zhongguo Zhongyao Zazhi, 15, 159–162 (1990).
Mayer, A. M., Polyphenol oxidase in plants-Recent progress.Phytochemistry, 26, 11–20 (1987).
Murga, R., Sanz, M. T., Beltran, S., and Cabezas, J. L., Solubility of some phenolic compounds contained in grape seeds, in supercritical carbon dioxide.J. Supercrit. Flu., 23, 113–121 (2002).
Nerya, O., Vaya, J., Musa, R., Izrael, S., Ben-Arie, R., and Tamir, S., Glabrene and isoliquiritigenin as tyrosinase inhibitors from Licorice Roots.J. Agric. Food Chem., 51, 1201–1207 (2003).
No, J. K., Soung, D. Y., Kim, Y. J., Shim, K. H., Jun, Y. S., Rhee, S. H., Yokozawa, T., and Chung, H. Y., Inhibition of tyrosinase by green tea components.Life Sci., 65, PL 241–246 (1999).
Perez-Bemal, A., Munoz-Perez, M. A., and Camacho, F., Management of facial hyperpigmentation.Am. J. Clin. Dermatol., 1, 261–268 (2000).
Rhee, Y. K., Han M. J., Park S. Y., and Kim D. H.,In vitro andIn vivo antitumor activity of the fruit body ofPhellinus linteus.Korean J. Food Sci. Technol., 32, 477–480 (2000).
Sang, S., Kikuzaki, H., Lapsley, K., Rosen, R. T., Nakatani, N., and Ho C. T., Sphingolipid and other constituents from almond nuts (Prunus amygdalus Batsch).J. Agric. Food Chem., 50, 4709–4712 (2002).
Sasaki, T., Arai, Y., Ikekawa, T., Chihara, G., and Fukuoka, E., Antitumor polysaccharides from some Polyporaceae,Gandderma applanatum (Pers.) Pat andPhellinus linteus (Berk,et Curt) Aoshima.Chem. Pharm. Bull., 19, 821–826 (1971).
Segel, I. H., Enzyme.In Biochemical calculations, John Wiley & Sons Inc., New York, 246 (1976).
Shibuya, H., Kawashima, K., Sakagami, M., Kawanishi, H., Shimomra, M., Ohashi, K., and Kitagawa, I., Sphingolipids and glycerolipids. I. Chemical structures and ionophoretic activities of soyacerebrosides I and II from soybean.Chem. Pharm. Bull., 38, 2933–2938 (1990).
Shimizu, M., Zenko, Y., Tanaka, R., Matsuzawa, T., and Morita, N., Studies on aldose reductase inhibitors from natural products. V. Active components of Hachimi-jio-gan (Kampo medicine).Chem. Pharm. Bull., 41, 1469–1471 (1993).
Shon, Y. H. and Nam, K. S., Antimutagenicity and induction of anticarcinogenic phase II enzymes by basidiomycetes.J. Ethnopharmacol., 77, 103–109 (2001).
Sitrin, R. D., Chan, G., Dingerdissen, J., DeBrosse, C., Mehta, R., Roberts, G., Rottschaefer, S., Staiger, D., Valenta, J., Snader, K. M., Stedman, R. J., and Hoover, J. R. E. Isolation and structure determination ofPachybasium cerebrosides which potentiate the antifungal activity of aculeacin.J. Antibiot., 41, 469–480 (1988).
Song, S. S., Kim, S. H., Sa, J. H., Jin, C., Lim, C. J., and Park, E. H., Anti-angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroomPhellinus linteus.J. Ethnopharmocol. 88, 113–116 (2003).
Stothers, J. B., Carbon-13 NMR spectroscopy. Academic Press: New York (1972).
Ulbricht, R. J., Northup, S. J., and Thomas, J. A., A review of 5- hydroxymethylfurfural (HMF) in parenteral solutions.Fundam. Appl. Toxicol., 4, 843–853 (1984).
Vesper, H., Schmelz, E. M., Nilolova-Karakashian, M. N., Dillehay, D. L., Lynch, D.V., and Merrill, A. H. Jr., Sphingolipids in food and the emerging importance of sphingolipids to nutrition.J. Nutr., 129, 1239–1250 (1997).
Wang, Y., Hasuma, T., Yano, Y., Morishima, Y., Matsui-Yuasa, I, and Otani, S., Induction of apoptosis in CTLL-2 cells by protocatechualdehyde.Anticancer Res., 21, 1095–1101 (2001).
Watanabe, K., Hayashi, H., and Mori, Y., Effect of a benzylidene derivative, a novel antirheumatic agent, on IL-1 production.Pharm. Res., 28, 57–72 (1993).
Webera, B., Hoescha, L., and Rasta, D. M., Protocatechualdehyde and other phenols as cell wall components of grapevine leaves.Phytochemistry, 40, 433–437 (1995).
Whitaker, J. R., Polyphenol oxidase.In Food Enzymes, Structure and Mechanism, Wong, D. W. S., Ed.; Chapman & Hall: New York, 271–307 (1995).
Xu, Y., Stokes, A. H., Freeman, W. M., Kumer, S. C., Vogt, B. A., and Vrana, K. E., Tyrosinase mRNA is expressed in human substantia nigra,Mol. Brain Res., 45, 159–162 (1997).
Yoshioka, A., Etoh, H., Yagi, A., Sakata, K., and Ina, K., Isolation of flavonoids and cerebrosides from the bark ofPrunus jamasakura as repellents against the blue mussel,Mytilus edulis.Agric. Biol. Chem., 54, 3355–3356 (1990).
Yue, J. M., Fan, C. Q., Xu, J., and Sun, H. D., Novel ceramides from the fungusLactarium volemus.J. Nat. Prod., 64, 1246–1248 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, H.S., Choi, J.H., Cho, W.K. et al. A sphingolipid and tyrosinase inhibitors from the fruiting body ofphellinus linteus . Arch Pharm Res 27, 742–750 (2004). https://doi.org/10.1007/BF02980143
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02980143