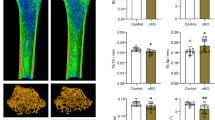
Abstract
Isoflavones have been a central subject in research on the natural phytoestrogens found inLeguminosae. Their effects on bone formation and remodeling are important in that they can act like estrogen by binding on estrogen receptors on the target cell surface. We, therefore, believed that isoflavones may help in the treatment of patients with estrogen deficiency disease such as estrogen replacement therapy (ERT) for osteoporosis. As commonly known, osteoporosis is one of the hormonal deficiency diseases, especially in menopausal women. When estrogen is no longer produced in the body a remarkable bone remodeling process occurs, and the associated events are regulated by growth factors in the osteoblast lineage. In the present study, we investigated whether isoflavones (Isocal) extracted fromSophorae fructus affect the growth factors IGF-I and TGF-P that have been known to be related with bone formation. In the study, we found that the active control (PIll) effectively enhanced the level of nitric oxide (NO) and growth factors, and thereby inhibited osteoclastogenesis. The most efficient concentration was 10−8% within five days, whereas the comparative control (soybean isoflavone) was not as effective even at a lower concentration. In conclusion, the products which contain enriched glucosidic isoflavone and nutrient supplements such as shark cartilage and calcium can be used for osteoporosis therapy by enhancing the production of IGF-I and TGF-β Furthermore, the NO produced through endothelial constitutive NO synthase (ecNOS) may play a role in inhibiting bone reabsorption.
Similar content being viewed by others
References
Adlercreutz, H., Phyto-oestrogens and cancer.Lancet Oncology, 3, 364–373 (2002).
Aguirre, J., Buttery, L., O’Shaughnessy, M., Afzal, F., Fernandez de Marticorena, I., Hukkanen, M., Huang, P., Manlntyre, I., and Polak, J., Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity.Am. J. Pathol., 158(1), 247–257 (2001).
Amonkar, M. M. and Mody, R., Developing profiles of postmenopausal women being prescribed oestrogen therapy to prevent osteoporosis.J. community Health, 27, 335–350 (2002).
Armpur, K. E. and Ralston, S. H., Estrogen upregulates endothelial constitutive nitric oxide synthase expression in human osteoblast-like cells.Endocrinology, 139, 799–802 (1998).
Aubin, J. E. and Bonnelye, E., Osteoprotegerin and its ligand: A new paradigm for regulation of osteoclastogenesis and bone reabsorption.Osteoporos. Int., 11, 905–913 (2000).
Baylink, D. J., Finkelman, R. D., and Moban, S., Growth factors to stimulate bone formation.J. Bone Miner. Res., 2(8), S565-S572 (1993).
Brandi, M. L., Hukkanen, M., Umeda, T., Moradi-Bidhendi, N., Bianchi, S., Gross, S. S., Polak, J. M., and Macintyre, I. Bidirectional regulation of osteoclast function by nitric oxide synthesis isofors.Proc. Natl. Acad. Sci. USA, 92, 2954–2958 (1995).
Bilezikian, J. P., Morishima, A., Bell, J., and Grumbach, M. M., Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency.N. Engl. J. Med., 339, 599–603 (1998).
Centrella, M., McCarthy, T., and Cannalis, E., Transforming growth factor-beta and remodeling of bone.J. Bone Joint Surg. Am., 73, 1418–1428 (1991).
Duncan, A. M., Phipps, W. R., and Kurzer, M. S., Phyto-oestrogen.Best Practice & Research Clinical Endocrinology and Metabolism, 17, 253–271 (2003).
Eastel, R., Treatment of postmenopausal osteoporosis.N. Engl. J. Med., 338, 736–746 (1998).
Greenfield, E. M., Bi, Y., and Miyauchi, A., Regulation of osteoclast activity.Life Sicence, 65, 1087–1102 (1999).
Joo, S. S., Chang, J. K., Park, J. H., Kang, H. C., and Lee, D. I., Immuno activation of lectin-conjugated praecoxin A on IL-6, IL-12 expression.Arch. Pharm. Res., 25, 954–963 (2002).
Kanamaru, Y., Takada, T., Saura, R., and Mizuno. K., Effect of nitric oxide on mouse clonal osteogenic cell, MC3T3-E1, proliferationin vitro.Kobe J. Med. Sci., 47, 1–11 (2001).
Klein-Nulend, J., Helfrich, M. H., Sterck, J. G., MacPherson, H., Joldersma, M., Ralson, S. H., Semeins, C. M., and Burger, E. H., Nitric oxide response to shear stress by human bone cell culture is endothelial nitric oxide synthase dependant.Biochem. Biophys. Res. Commun., 250, 108–114 (1998).
Lian, J., Stein, G., Canalis, E., Robey, P., and Boskey, A., Bone formation: osteoblast lineage cells, growth factors, matrix proteins, and the mineralization process in. In Favus M, ed. Primer on the metabolic bone disease and disorders of mineral metabolism. Vol. 1. Philadelphia: Lippincott Williams and Wilkins (1999).
Messina, M. and Messina, V., Soyfood, soybean isoflavone, abd bone health: a brief overview.J. Renal Nutrition, 10, 63–68 (2000).
Mundy, G., Bone modeling. In: Favus M., ed. Primer on the metabolic bone disease and disorders of mineral metabolism. Vol. 1. 4th ed. Philadelphia, Lippincott Williams and Wilkins (1999).
Mundy, G. R., Bone remodeling and its disorders (ed. M. Dunits). London, UK (1995).
Nakagawa, N., Kinosaki, M., Yamaguchi, K., Shima, N., Yasuda, H., Yano, K., Morinaga, T., and Higashio, K., RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis.Biochem. Biophys. Res. Commun., 253, 395–400 (1998).
Pacifici, R., Cytokines, estrogen and postmenopausal osteoporosis-the second decade.Endocrinology, 139, 2659–2661 (1998).
Raisz, L. G., The osteoporosis revolution.Ann. Intern. Med., 126, 458–462 (1997).
Riggs, B. L., Khosia, S., and Melton, L. J., A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men.J. Bone Miner. Res., 13, 763–773 (1998).
Setchell, K. D., Abstract presented at: Second international symposium on the role of soy in preventing and treating chronic disease. Brussel Belgium (1996).
Swolin-Eide, D. and Ohlsson, C., Effects of Cortisol on the expression of interleukin-6 and interleukin-1β in human osteoblast like cells.J. Endocrinology., 156, 107–114 (1998).
Udagawa, N., Takahashi, N., Matsuzaki, K., Jimi, E., Tsurukai, T., Itoh, K., Nakagawa, H., Yasuda, H., Goto, M., Tsuda, E., Higashio, K., Martin, T. J., and Suda, T., Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand.Bone, 5, 517–523 (1999).
van’t Hof, R. J. and Ralston, S. H., Nitric oxide and bone.Immunology, 103, 255–261 (2001).
Warren, M. P., Shortle, B., and Dominguez, J. E., Use of alternative therapies in menopause.Best Practice & Research Clinical Obstetrics and Gynaecology, 16(3), 411–448 (2002).
Wimalawansa, S. J., De Marco, G., Gangula, P., and Yallampalli, Nitric oxide donor alleviates ovariectomy-induced bone loss.Bone, 18, 301–304 (1996).
Yamaguchi, M. and Gao, Y. H., Inhibitory effect of genistein on bone reabsorption in tissue culture.Biochem. Phamacol., 55, 71–76 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joo, SS., Won, TJ., Kang, HC. et al. Isoflavones extracted fromsophorae fructus upregulate IGF-I and TGF-β and inhibit osteoclastogenesis in rat born marrow cells. Arch Pharm Res 27, 99–105 (2004). https://doi.org/10.1007/BF02980054
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02980054