Abstract
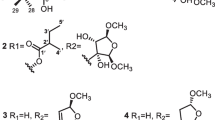
Two new furanolignans (3, 5), together with three known lignans (1, 2, 4), were isolated from the stem ofLindera obtusiloba (Lauraceae). The structures of the compounds were determined as actifolin (1), pluviatilol (2), 5,6-dihydroxymatairesinol (3), (+)-syringaresinol (4), and (+)-9′-O-trans-feruloyl-5,5′-dimethoxylariciresinol (5) on the basis of physicochemical and spectroscopic evidences. Compounds1, 2, 3, and5 showed cytotoxicity against a small panel of human tumor cell lines with ED50 values of 3.40∼19.27 μg/ml.
Similar content being viewed by others
References Cited
Corrie, J. E. T., Green, G. H. and Taylor, W. C., The chemical constituents of AustralianZanthoxylum species.Aust. J. Chem., 23, 133–145 (1970).
Deyama, T., Ikawa, T., Kitagawa, S. and Nishibe, S., The constituents of Eucommia ulmoides Oliv. V. Isolation of dihydroxydehydrodiconiferyl alcoho isomers and phenolic compounds.Chem. Pharm. Bull., 35, 1785–1789 (1987).
Hsiao, J. J. and Chiang, H. C., Lignans from the wood ofAralia bipinnata.Phytochemistry, 39, 899–902 (1995).
Kinjo, J., Higuchi, H., Fukui, K. and Nohara, T., Lignoids from Albizziae Cortex II. A biodegradation pathway of syringaresinol.Chem. Pharm. Bull., 39, 2952–2955 (1991).
Komae, H. and Hayashi, H., Phytosterols of the trunks ofLindera obtusiloba.Phytochemistry, 11, 1182 (1972).
Macrae, W. D. and Towers, G. H. N., Non-alkaloidal constituents ofVirola elongata Bark.Phytochemistry, 24, 561–566 (1985).
Nishibe, S., Chiba, M. Sakushima, A., Hisada, S., Yamanouchi, S., Takido, M., Sankawa, U. and Sakakibara, A., Introduction of an alcoholic hydroxyl group into 2,3-dibenzylbutyrolactone lignans with oxidizing agents and carbon-13 nuclear magnetic resonance spectra of the oxidation products.Chem. Pharm. Bull., 28, 850–860 (1980).
Niwa, M., Iguchi, M. and Yamamura, S., Three new obtusilactones fromLindera obtusiloba Blume.Chemistry Letters, 655–658 (1975a).
Niwa, M., Iguchi, M. and Yamamura, S., The structures of C17-obtusilactone dimer and two C21-obtusilactones.Tetrahedron Letters, 49, 4395–4398 (1975b).
Skehan, P., Storeng, R., Scudiero, D., Monks, A., Mc-Mahon, J., Vistica, D., Warren, J. T., Bokesch, H., Kenney, S. and Boyd, M. R., New colorimetric cytotoxicity assay for anticancer-drug screening.J. Natl. Cancer Inst. 82, 1107–1112 (1990).
Subbaraju, G. V., Kumar, K. K. K., Raju, B. L. and Pllai, K. R., Justiciresinol, a new furanoid lignan fromJusticia glauca.J. Na. Prod., 54, 1639–1641 (1991).
Taffrout, M., Rouessac, F. and Robin, J.-P., Isolement du prestegane B à partir de Steganotaenia araliacea Hochst., premier lignane bis-(meta-hydroxy-benzyl)-butanolide d'origine vè gè table.Tetrahedron Letters, 25, 4127–4128 (1984).
Tanaka, H., Nakamura, T., Ichino, K. and Ito, K., A lignan fromActinodaphne longifolia Phytochemistry, 28, 952–954 (1989).
Tanoguchi, M., Hosono, E., Kitaoka, M., Arimoto, M. and Yamaguchi, H., Studies on the constituents of the seeds ofHernandia ovigera L. IX. Identification of two dibenzylbutyrolactone-type lignans and an attempt of conversion into phenyltetralin-type lignan.Chem. Pharm. Bull., 39, 1873–1876 (1991).
Yook, C. S., Medicinal Plants of Korea. Academy Publishing Co., Seoul, Korea, p.184, 1989.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwon, H.C., Choi, S.U., Lee, J.O. et al. Two new lignans fromLindera obtusiloba blume. Arch Pharm Res 22, 417–422 (1999). https://doi.org/10.1007/BF02979069
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02979069