Abstract
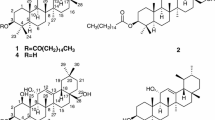
The chromatographic separation of the MeOH extract from the aerial parts ofSyneilesis palmata led to the isolation of a new sesquiterpene glycoside4, together with four known compounds. Their structures were characterized to be 4β,5β-epoxy-caryophill-8,(15)-ene (1), 3β-hydroxy-gultin-5-ene (2), 4α,5β-dihydroxy-caryophill-8,(15)-ene (3), (-)-oplopan-4-one-10-α-O-β-D-glucose (4) and 3-hexenyl-1-O-β-D-glucopyranose (5), based on spectroscopic and chemical methods. Compound2 showed moderate cytotoxicity against five human tumor cell linesin vitro with its ED50 values ranging from 5.90∼10.83 μg/mL.
Similar content being viewed by others
References
Alberto, M. J., Sanz-Cervera, J. F., Sancenon, F., Jakuovic J., Rustaiyan, A., and Mohamadi, F., Oplopanone derivatives and monoterpene glycosides fromArtemisia sieberi.Phytochemistry, 34(4), 1061–1065 (1993).
An, D. K., Illastated Book of Korean medicinal Herbs, Kyohak-Sa, p 347 (1998).
Antionio, G. G., Esteban, A. F., and Angel, G., R., Triterpenes fromMaytenus Horrida.Phytochemistry, 26(10), 2785–2788 (1987).
Bolhmann, F. and Grenz, M., Terpeneglucoside ausSyneilesis aconitifolia.Phytochemistry, 16, 1057–1059 (1977).
Bolhmann, F. and Zdero, C., Neue furanoeremophilane und andere sesquiterpene aus vertretern gattungEurops.Phytochemistry, 17, 1135–1153 (1978).
Choi, S. Z., Kwon, H. C., Choi, S. U., and Lee, K. R., Rive New Labdane Diterpenes fromAster oharai.J. Nat. Prod., 65(8), 1102–1106 (2002).
Choi, S. Z., Lee, S. O., Choi, S. U., and Lee, K. R., A new sesquiterpene hydroperoxide from the aerial parts ofAster oharai.Arch. Pharm. Res., 26(7), 521–525 (2003).
Collado, I. G., Hanson, J. R., Hitchcock, P. B., and Macias-Sanchez, A. J., Streochemistry of epoxidation of some caryophyllenols.J. Org. Chem., 62, 1965–1969 (1997).
Kitagawa, I., Cui, Z., Son, B. W., Kobayashi, S., and Kyogoku, Y., Marine natural products. XVII. Nephtheoxydiol, a new cytotoxic hydroperoxy-germacrane, and related sesquiter-penoids from Okinawan coral ofNephthea sp.(Nephtheidae).Chem. Pharm. Bull., 35(1), 124–135 (1987).
Kuroda, C., Murae, T., Tada, M., Nagano, H., and Takahashi, T., New 14-oxofuranoeremophilanes and pelated sesquiterpenes fromSyneilesis palmata (Thunb.) Maxim.Chem. Lett., 1313–1316 (1978).
Kwon, H. C., Cho, O. R., Lee, K. C., and Lee, K. R., Cerebrosides and terpene glycosides from the root ofAster scaber.Arch. Pharm. Res., 26(2), 132–137 (2003).
Lee Y. N., Flora of Korea 3rd edition, Kyohak-Sa, p 814 (1998).
Manabu, H. and Tsutomu, F., Syneilesine, a new pyrrolizine alkaloid fromSyneilesis palmata.Tetrahedron Lett., 41, 3657–3660(1974).
Mizutani, K., Yuda, M., Tanaka, O., Saruwatari, Y. I., Fuwa, T., Jia, M. R., Ling, Y. K., and Pu, X. F., Chemical studies on Chinese traditional medicine, Dangshen. I. isolation of (Z)-3-and (E)-2-hexenyl β-D-glucosides.Chem. Pharm. Bull., 36(7), 2689–2690 (1988).
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J. T., Bokesch, H., Kenney, S., and Boyd, M. R., New colorimetric cytotoxicity assay for anticancer-drug screening.J. Natl. Cancer Inst., 82, 1107–1112 (1990).
Sung, T. V., Steffan, B., Steglich, W., Klebe, G., and Adam, G., Sesquiterpenoids from the roots ofHomalomena aromatica.Phytochemistry, 31(10), 3515–3520 (1992).
Tatarova, L. E., Korchabina, D. V., and Barkhash, V. A., Reactions of caryophyllene 4β,5α-epoxide with carbonyl compounds on clay.Rus. J. Org. Chem., 38(4), 519–524 (2002).
Takeda, K., Minato, H., and Ishikawa, M., Studies on sesquiter-penoids-XII structure and absolute configuration of oplopanone, a new sesquiterpene fromOplopanax japonicus (Nakai) NAKAI.Tetrahedron suppl., 7, 219–225 (1965).
Vignon, M. R. and Vottero, J. A., RMN13C: Sur I’utilisation des esters pour I’attribution des carbones des molecules glucidiques.Tetrahedron Lett., 28, 2445–2448 (1976).
Ye, Q., Qin, G., and Zhao, W., Immunomodulatory sesquiterpene glycosides fromDendrobium nobile.Phytochemistry, 61, 885–890 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, K.H., Choi, S.U. & Lee, K.R. Sesquiterpenes fromSyneilesis palmata and their cytotoxicity against human cancer cell linesin vitro . Arch Pharm Res 28, 280–284 (2005). https://doi.org/10.1007/BF02977792
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977792