Abstract
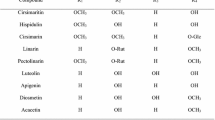
Seven flavonoids were isolated from the whole plants and fruits ofCayratia japonica through the activity-guided isolation of a methanol extract using a monoamine oxidase (MAO) inhibition assay as a monitor. The chemical structures of the isolates were assigned as apigenin-7-O-β-D-glucuronopyranoside (1), apigenin (2), luteolin (3), luteolin-7-O-β-D-glucopyranoside (4), (+)-dihydroquercetin (taxifolin) (5), (+)-dihydrokaempferol (aromadendrin) (6) and quercetin (7). Among the isolated compounds, flavones such as apigenin (2) and luteolin (3), as well as the flavonol, quercetin (7) showed potent inhibitory effects against the MAO activity with IC50 values of 6.5, 22.6, and 31.6 μM, respectively. However, the flavone glycosides, apigenin-7-O-β-D-glucuronopyranoside (1) and luteolin-7-O-β-D-glucopyranoside (4), showed mild MAO inhibition (IC50 values: 81.7 and 118.6 μM, respectively). The flavanonol derivatives, taxifolin (5) and aromadendrin (6), also showed weak inhibition (IC50 values: 154.7 and 153.1 μM, respectively). Furthermore, quercetin (7) had a more potent inhibitory effect on MAO-A (IC60 value: 2.8 μM) than MAO-B (IC50 value: 90.0 μ.M). Apigenin (2) and luteolin (3) also preferentially inhibited MAO-A (IC50 values: 1.7 and 4.9 μM, respectively) compared with MAO-B (IC50 values: 12.8 and 59.7 μM, respectively).
Similar content being viewed by others
References
Abell, C. W. and Kwan, S. W., Molecular characterization of monoamine oxidases A and B.Prog. Nucleic Acid Res. Mol. Biol., 65, 129–156(2001).
Agrawal, P. K., Agarwal, S. K., Rastogi, R. P., and Osterdahal, B. G, Dihydroflavanonols fromCedrus deodara, A13C NMR study.Planta Med., 43, 82–85(1981).
Chimenti, F., Cottiglia, R., Bonsignore, L., Casu, L., Casu, M., Floris, C., Secci, D., Bolasco, A., Chimenti, P., Granese, A., Befani, O., Turini, P., Alcaro, S., Ortuso, F., Trombetta, G., Loizzo, A., and Guarino, I., Quercetin as the active principle ofHypericum hircinum exerts a selective inhibitory activity against MAO-A: extraction, biological analysis, and computational study.J. Nat. Prod., 69, 945–949 (2006).
Choe, S. G, Hwang, B. Y, Kim, M. S., Oh, G. J., Lee, K. S., and Ro, J. S., Chemical components ofRumex acetosella.Kor. J. Pharmacogn., 29, 209–216 (1998).
Han, Y. N., Noh, D. B., and Han, D. S., Studies on the monoamine oxidase inhibitors of medicinal plants I. isolation of MAO-B inhibitors fromChrysanthemum indicum.Arch. Pharm. Res., 10, 142–147(1987).
Hase, T., Ohtani, K., Kasai, R., Yamasaki, K., and Picheansoonthon, C, Revised structure for hortensin, a flavonoid fromMillingtonia hortensis.Phytochemistry, 40, 287–290(1995).
He, F. Y. and Ling, L. Q., Phytochemical study ofCayratia japonica.Zhong Cheng Yao Yan Jiu, 4, 30–32 (1987) (Chinese).
Henchcliffe, C., Schumacher, H. C., and Burgut, F. T., Recent advances in Parkinson’s disease therapy: use of monoamine oxidase inhibitors.Expert. Rev. Neurother, 5, 811–821 (2005).
Ishikura, N. and Shibata, M., Cayratinin, a new anthocyanin from the pericarp ofCayratia japonica.Shokubutsugaku Zasshi, 83, 179–183(1970).
Jung, B. S. and Shin, M. K., Encyclopedia of illustrated Korean natural drugs. Young Lim Sa, Seoul, p. 300 (1990).
Kim, J. S., Kang, S. S., Lee, M. W., and Kim, O. K., Isolation of flavonoids from the leaves ofAralia continentalis.Kor. J. Pharmacogn., 26, 239–243 (1995).
Kraml, M., A rapid microfluorimetric determination of monoamine oxidase.Biochem. Pharmacol., 14, 1684–1686(1965).
Lee, E. H., Kim, H. J., Song, Y S., Jin, C, Lee, K. T., Cho, J., and Lee, Y S., Constituents of the stems and fruits ofOpuntia ficus-indica vansaboten.Arch. Pharm. Res., 26, 1018–1023(2003).
Lee, S. J., Chung, H. Y, Lee, I. K., Oh, S. U., and Yoo, I. D., Phenolics with inhibitory activity on mouse brain monoamine oxidase (MAO) from whole parts ofArtemisia vulgaris L (Mugwort).Food Sci. Biotechnol., 9, 179–182 (2000).
Lee, S. S., Kai, M., and Lee, M. K., Effects of natural isoquinoline alkaloids on monoamine oxidase activity in mouse brain: inhibition by berberine and palmatine.Med. Sci. Res., 27, 749–751 (1999).
Naoi, M., Matsuura, S, Parvez, H, Takahashi, T, Hirata, Y, Minami, M., and Nagatsu, T, Oxidation of N-methyl-1, 2, 3, 4- tetrahydroisoquinoline into the N-methyl- isoquinolinium ion by monoamine oxidase.J. Neurochem., 52, 653–655 (1989).
Parmar, V. S., Bisht, K. S., Sharma, S. K., Jain, R., Taneja, P., Singh, S., Simonsen, O., and Boll, P. M., Highly oxygenated bioactive flavones fromTamarix.Phytochemistry, 36, 507- 511 (1994).
Ryu, S. Y., Han, Y N., and Han, B. H., Monoamine oxidase-A inhibitors from medicinal plants.Arch. Pharm. Res., 11, 230- 239(1988).
Shih, J. C., Chen, K., and Ridd, M. J., Monoamine oxidase: from genes to behavior.Annu. Rev. Neurosci., 22, 197–217 (1999).
Sloley, B. D., Urichuk, L. J., Morley, P., Durkin, J., Shan, J. J., Pang, P. K., and Coutts, R. T., Identification of kaempferol as a monoamine oxidase inhibitor and potential neuroprotectant in extracts ofGinkgo biloba leaves.J. Pharm. Pharmacol., 52, 451–459(2000).
Sonobe, T., Taniguchi, M., Omura, T, Kasai, H., Saito, Y., Kamano, N., and Nakayama, T., Biologically active polyphenols fromCayratia japonica of the Vitaceae family, manufacture and pharmaceutical preparations.Japan Kokai Tokkyo Koho, 10, 226 668(1998).
Thull, U. and Testa, B., Screening of unsubstituted cyclic compounds as inhibitors of monoamine oxidases.Biochem. Pharmacol., 47, 2307–2310 (1994).
Youdim, M. B., Edmondson, D., and Tipton, K. R, The therapeutic potential of monoamine oxidase inhibitors.Nat. Rev. Neurosci., 7, 295–309 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, X.H., Hong, S.S., Hwang, J.S. et al. Monoamine oxidase inhibitory components fromCayratia japonica . Arch Pharm Res 30, 13–17 (2007). https://doi.org/10.1007/BF02977772
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977772