Abstract
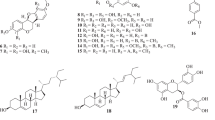
Twelve compounds with lipid peroxidation inhibitory activity were isolated from the stem bark ofE. globulus. Their structures were assigned as a new aromatic monoterpene (1) and eleven known compounds, pinoresinol (2), vomifoliol (3), 3,4,5-trimethoxyphenol 1-O-β-D-(6′-O-galloyl)glucopyranoside (4), methyl gallate (5), rhamnazin (6), rhamnetin (7), eriodictyol (8), quercetin (9), taxifolin (10), engelitin (11), and catechin (12) on the basis of UV, mass, and NMR spectroscopic analyses. These compounds except vomifoliol significantly inhibited lipid peroxidation in rat liver microsome.
Similar content being viewed by others
References
Ghisalberti, E. L., Bioactive acylphloroglucinol derivatives fromEucalyptus species.Phytochemistry, 41, 7–22 (1996).
Herderich, M., Feser, W. and Schreier, P., Vomifoliol 9-O-β-D-glucopyranosyl-4-O-β-D-xylopyranosyl-6-O-β-D-glucopyranoside: a trisaccharide glycoside from apple fruit.Phytochemistry, 31, 895–897 (1992).
Hogeboom, G. H., General methods for the isolation of liver cell components: Fraction of cell components of animal tissues.Meth. Enzymol., 1, 16–19 (1965).
Ishimaru, K., Nonaka, G. and Nishioka, I., Phenolic glucoside gallates fromQuercus mongolica andQ. acutissima.Phytochemistry, 26, 1147–1152 (1987).
Kozuka, M., Sawada, T., Kasahara, F., Mizuta, E., Amano, T., Komiya, T. and Goto, M., The granulation-inhibiting principles fromEucalyptus globulus Labill. II. the structures of euglobal-Ia1,-Ia2,-Ib,-Ic,-IIa,-IIb and-IIc.Chem. Pharm. Bull., 30, 1952–1963 (1982a).
Kozuka, M., Sawada, T., Mizuta, E., Kasahara, F., Amano, T., Komiya, T. and Goto, M., The granulation-inhibiting principles fromEucalyptus globulus Labill. III. the structures of euglobal-III,-IVb and-VII.Chem. Pharm. Bull., 30, 1964–1973 (1982b).
Nishizawa, M., Emura, M., Kan, Y., Yamada, H., Ogawa, K. and Hamanaka, N., Macrocarpals: HIV-RTase inhibitors ofEucalyptus globulus.Tetrahedron Lett., 33, 2983–2986 (1992).
Nonaka, G., Nishimura, H. and Nishioka, I., Tannins and related compounds. IV. seven new phenol glucoside gallates fromQuercus stenophylla Makino.Chem. Pharm. Bull., 30, 2061–2067 (1982).
Ohkawa, H., Ohishi, N. and Yagi, K., Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.Anal. Biochem., 95, 351–358 (1979).
Okuyama, E., Suzumura, K. and Yamazaki, M., Pharmacologically active components of Todopon Puok (Fagraea racemosa), a medicinal plant from Borneo.Chem. Pharm. Bull., 43, 2200–2204 (1995).
Osawa, K., Yasuda, H., Morita, H., Takeya, K. and Itokawa, H., Eucalyptone fromEucalyptus globulus.Phytochemistry, 40, 183–184 (1995).
Patwardhan, S. A. and Gupta, A. S., Aromatic monoterpenes fromLavandula gibsonii.Phytochemistry, 22, 2080–2081 (1983).
Piovetti, L., Combaut, G. and Diara, A., Monoterpenes et sesquiterpenes oxygenes deCupressus dupreziana.Phytochemistry, 19, 2117–2120 (1980).
Santos, G. G., Alves, J. C. N., Rodilla, J. M. L., Duarte, A. P., Lithgow, A. M. and Urones, J. G., Terpenoids and other constituents ofEucalyptus globulus.Phytochemistry, 44, 1309–1312 (1997).
Wu, T.-S., Niwa, M., Furukawa, H. and Kuoh, C.-S., Eupatriol, a new monoterpene fromEupatorium tashiroi Hayata.Chem. Pharm. Bull., 33, 4005–4006 (1985).
Yamakoshi, Y., Murata, M., Shimizu, A. and Homma, S., Isolation and characterization of macrocarpals B-G antibacterial compounds fromEucalyptus macrocarpa.Biosci. Biotech. Biochem., 56, 1570–1576 (1992).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yun, BS., Lee, IK., Kim, JP. et al. Lipid peroxidation inhibitory activity of some constituents isolated from the stem bark ofEucalyptus globulus . Arch Pharm Res 23, 147–150 (2000). https://doi.org/10.1007/BF02975503
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02975503