Abstract
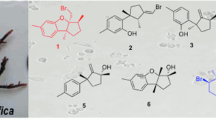
A novel and two known bioactive mono-tetrahydrofuran (THF) annonaceous acetogenins, annomocherin (1), annonacin (2) and annomontacin (3), have been isolated from the fractionated ethanolic extracts of the seeds ofAnnona cherimolia, guided by the brine shrimp lethality test (BST). Their structures were elucidated on the basis of spectroscopic and chemical methods. All compounds have a relative stereochemistry ofthreo/trans/threo for the mono-THF ring with two flanking hydroxyls. Compound1 has a double bond at C-23/ 24 of aliphatic chain. Compound1 was isolated from natural sources for the first time, and was named annomocherin. Two known Compounds2 and3 which have never been isolated from this species before, were obtained. Compound1 exhibited potent and selective cytotoxicities against the breast carcinoma (MCF-7) and kidney carcinoma (A-498) cell lines with 100 to 1,000 times the potency of adriamycin. In brine shrimp lethality test (BST),1–3 exhibited cytotoxicity.
Similar content being viewed by others
References
Alali, F., Liu, X. X., McLaughlin, J. L., Annonaceous acetogenins: Recent progress.J. Nat. Prod., 62, 504–540 (1999).
Alkofahi, A., Rupprecht, J. K., Smith, D. L., Chang, C. J., and McLaughlin, J. L., Coniothalamicin and annonacin: Bioactive acetogenins fromGoniothalamus giganteus (Annonaceae).Experientia, 44, 83–85 (1988).
Araya, H., Hara, N., Fujimoto, Y., Srivastava, A., and Sahai, M., Squamosten-A, a novel mono-tetrahydrofu- ranic acetogenin with a double bond in the hydrocarbon chain, fromAnnona squamosa L.Chem. Pharm. Bull., 42, 388–391 (1994).
Barriga, H. C.Flora Medicinal de Colombia. Vol. 1, Botánica, Bogatá, p. 340, 1974.
Born, L., Lieb, F., Moescher, J. P., Nonfon, H. F., Soller, R., and Wendish, D. The relative configuration of acetogenins isolated fromAnnona squamosa: annonin I (squamocin) and annonin.Planta Med., 56, 312–316 (1990).
Colman-Saizarbitoria, T., Zambrano, J. Ferrigni, N. R., Gu, Z. M., Ng, J. H., Smith, D. L., and McLaughlin, J. L., Bioactive annonaceous acetogenins from the bark ofXylopia aromatica.J. Nat. Prod., 57, 486–493 (1994).
Colman-Saizarbitoria, T., Johnson, H. A., Alali, F. Q., Hopp, D. C. Rogers, L. L., and McLaughlin, J. L., Annojahnin formAnnona jahnii: A possible precursor of mono-THF acetogenins.Phytochemistry, 49, 1609–1616 (1998).
Corters, D. Myint, S. H., Laurens, A., Hocquemiller, R. Leboeuf, M., and Cave A., Corossolone et corossoline, deux nouvelles γ-lactone fromAnnona muricata.Heterocycles, 31, 861–867 (1991).
Fang, X. P., Rieser, M. J., Gu, Z. -M., Zhao, G. -X., and McLaughlin, J. L., Annonaceous acetogenins: an updated review.Phytochem. Anal., 4, 27–67 (1993).
Fies, R. E., “Annonaceae”, in “Die Natülichen Pflanzenfamilien”, Engler, A., Prantl, K. (Eds.) 2nded., Dunker and Humbolt, Berlin, Vol. 17, 1959.
Fogh, J. and Trempe, G.,New human tumor cell lines In human tumor cells; Fogh, J., Ed.; Plenum Press: New York, pp 115–119 (1973).
Giard, D. L., Aronson, S. A., Todaro, G. J., Arnstein, P., Kersey, J. H., Dosik, H., and Parks, W. P., In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors.J. Natl. Cancer Inst. 51, 1417–1423 (1973).
Gu. Z. M., Zeng, L., and Fang, X. P., Colman-Saizarbitoria, T. Huo, M., McLaughlin, J. L., Determining absolute configurations of stereocenters in annonaceous acetogenin through formaldehyde acetal derivatives and Mosher ester methodology.J. Org. Chem., 59, 5162–5172 (1994a).
Gu. Z. M., Fang, X. P., Zeng, L., Song, R., Ng, J. H., and Wood, K. V., Gonionenin: a new cytotoxic annonaceous acetogenin fromGoniothalamus giganteus and the conversion of mono-THF acetogenins to bis-THF acetogenins.J. Org. Chem. 59, 3472–3479 (1994b).
Harmange, J. C., Figadere, B., and Cave, A., Stereo-controlled synthesis of 2,5-linked monotetrahydrofuran units of acetogenins.Tetrahedron Lett. 33, 5749–5752 (1992).
Hopp, D. C., Zeng, L., Gu, Z. M., Kozlowski, J. F., and McLaughlin, J. L., Novel mono-tetrahydrofuran ring acetogenins, from the barkof Annona squamosa, showing cytotoxic selectivities for the human pancreatic carcinoma cell line, PACA-2.J. Nat. Prod., 60, 581–585 (1997).
Hoye, T. R., Hanson, P. R., Hasenwinkel, L. E., Ranirez, E. A., and Zhuang, Z., Stereostructural studies on the 4-hydroxylated annonaceous acetogenins; Synthesis of model butenolides of known relative and absolute configuration involving an intriguing translactonization reaction.Tetrahedron Lett., 35, 8525–8528 (1994a).
Hoye, T. R., Hanson, P. R., Hasenwinkel, L. E., Ranirez, E. A., and Zhuang, Z., Stereostructural studies on the 4-hydroxylated annonaceous acetogenins; A novel use of mosher ester data for determining relative configuration [between C(4) and C(36)].Tetrahedron Lett., 35, 8529–8532 (1994b).
Joldad, S. D., Hoffmann, J. J. Cole, J. R., Barry, C. E., Bates, R. B., Linz, G. S., and Konig, W. A., Uvaricin, a new antitumor agent fromUvaria accuminata (Annonaceae).J. Nat. Prod., 48, 644–645 (1985).
Jossang, A., Dubaele, B., and Cave, A., Deux nouvelles acetogenines monotetrahydrofuraniques cytotoxiques: 1′annonacine et la montanacine.Tetrahedron Lett., 31, 1861 (1990).
Jossang, A., Dubaele, B., Cave, A., Bartoli, M. H., and Beriel, H., Annomontacin: une nouvelle acetogenine γ-lactone-monotetrahydrofuranique cytotoxique de 1′Annona montana.J. Nat. Prod., 54, 967–971 (1991).
Kaighn, M. E., Narayan, K. S., Ohnuki, Y., Lechner, J. F., and Jones, L. W., Establishment and characterization of a human prostatic carcinoma cell line (PC-3).Invest. Urol., 17, 16–23 (1979).
Kim, D. H. and Woo, M. H., Corrosolin and compound- 2: Cytotoxic annonaceous acetogenins from the seeds ofAnnona cherimolia.Yakhak Hoeji, 43, 584–590 (1999).
Kim, D. H., Ma, E. S., Suk, K. D., Son, J. K., Lee, J. S., and Woo, M. H., Annomolin and annocherimolin, New cytotoxic annonaceous acetogenins fromAnnona cherimolia Seeds.J. Nat. Prod. 64, 502–506 (2001).
Laprevote, O., Girard, C., and Das, B. C., Formation of gas-phase lithium complexes from acetogenins and their analysis by fast atom bombardment mass spectrometry.Tetrahedron Lett., 33, 5237–5240 (1992).
McLaughlin, J. L., “Methods in Plant Biochemistry”, Hostettmann, K. (Ed.), Academic Press, London, Vol. 6, pp. 1–35, 1991.
Meyer, B. N., Ferrigni, N. R., Putnam, J. E., Jacobson, L. B., Nichols, D. E., and McLaughlin, J. L., Brine shrimp: a convenient general bioassay for active plant constituents.Planta Med., 45, 31–34 (1982).
Morre, J. D., Decabo, R, D., Farley, C., Oberlies, N. H., and McLaughlin, J. L., Mode of action of bullatacin, a potent antitumor acetogenin: inhibition of NADH oxidase activity of HELA and HL-60, but not liver, plasma membranes.Life Sci., 56, 343–348 (1995).
Mosher, H. S., and Dale, J. A. Nuclear magnetic resonance enantiomer reagents. Configurational correlationsvia nuclear magnetic resonance chemical shifts of diastereomeric mandelate,O-methylmandelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters.J. Am. Chem. Soc., 95, 512–519 (1973).
Rieser, M. J., Hui, Y. H., Rupprecht, J. K., Kozlowshi, J. F., Wood, K. V., McLaughlin, J. L., Hanson, P. R., Zhuang, A., and Hoye, T. R., Determination of absolute configuration of sterogenic carbinol centers in annonaceous acetogenins by1H- and19F-NMR analysis of mosher ester derivatives.J. Am. Chem. Soc., 144, 10203–10213 (1992).
Rupprecht, J. K., Hui, Y. H., and McLaughlin, J. L., Annonaceous acetogenins: a review.J. Nat. Prod. 53, 237–278 (1990).
Sahai, M., Singh, S., Singh, M., Gupta, Y. K., Akashi, S., Yuji, R., Hirayama, K., Asaki, H., Araya, H., Hara, N., Eguchi, T., Kakinuma, K., and Fujimoto, Y., Annonaceous acetogenins from the seeds ofAnnona squamosa. Adjacent bis-tetrahydrofuranic acetogenins.Chem. Pharm. Bull., 42, 1163–1174 (1994).
Soule, H. D., Vazquez, J., Long, A., Albert, S., and Brennam, M., A human cell line from a pleural effusion derived from a breast carcinoma.J. Natl. Cancer Inst, 51, 1409–1416. (1973).
Woo, M. H., Zeng, L., and McLaughlin, J. L., Asitribin and asiminenins A and B, novel bioactive Annonaceous acetogneins from the seeds ofAsimina triloba.Heterocycles, 41, 1731–1742 (1995).
Woo, M. H., Kim, D. H., Fotopoulos, S. S., and McLaughlin, J. L., Annocherin and (2, 4)-cis-andtrans-annocherinones: monotetrahydrofuran Annonaceous aceto-genins with a C-7 carbonyl group fromAnnona cherimolia seeds.J. Nat. Prod., 62, 1250–1255 (1999).
Woo, M. H., Chung, S. O., and Kim, D. H.,cis-Annonacin and (2,4)-cis- andtrans-isoannonacins: cytotoxic monotetrahydrofuran annonaceous acetogenins from the seeds ofAnnona cherimolia.Arch. Pharm. Res., 22, 524–528 (1999).
Yunis, A. A., Arimura, G. K., and Russin, D., Human pancreatic carcinoma (MIA PaCa-2) in continuing culture: sensitivity to asparaginase.Int. J. Cancer, 19, 128–135 (1977).
Zeng, L., Ye, Q., Oberlies, N. H., Shi, G., Gu, Z. -M., He, K., and McLaughlin, J. L., Recent Advances in Annonaceous acetogenins.Nat. Prod. Rep., 13, 275–306 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, D.H., Son, J.K. & Woo, M.H. Annomocherin, Annonacin and Annomontacin: a novel and two known Bioactive mono-tetrahydrofuran annonaceous acetogenins fromAnnona cherimolia seeds. Arch Pharm Res 24, 300–306 (2001). https://doi.org/10.1007/BF02975096
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02975096