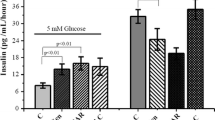
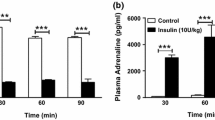
Abstract
Hypoglycemic action of brazilin was found to be based on the improvement of peripheral glucose utility, and this action might be correlated with the insulin action pathway. In the present study we investigated the effect of brazilin on the insulin receptor autophosphorylation, protein kinase C (PKC), protein phosphatase and insulin receptor serine kinase in order to confirm whether the hypoglycemic mechanism is concerned with insulin action pathway. Brazilin was found to inhibit PKC and insulin receptor serine kinase, which are involved in the regulation of insulin signal pathway. But any significant effect was not shown on insulin receptor tyrosine kinase activity, autophosphorylation and phosphatase activity. These findings suggest that brazilin might enhance insulin receptor function by decreasing serine phosphorylation, which might mediate hypoglycemic effect of brazilin
Similar content being viewed by others
References Cited
Baltensperger K., Rewis R. E., Woon C. W., Vissavajjhala P., Ross A.H. and Czech M. P., Catalysis of serine and tyrosine autophosphorylation by the human insulin receptor.Proc. Natl. Acad. Sci. USA., 89, 7885–7889 (1992).
Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A. and Rosen O. M., Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin.J. Biol. Chem., 262, 1842–1847 (1987).
Cuatrecasas P., Isolation of the insulin receptor of liver and fat cell membranes.Proc. Natl. Acad. Sci. USA, 69, 318–322 (1972).
Duronio V. and Jacobs S., The effect of protein kinase-C inhibition on insulin receptor phosphorylation.Endocrinology, 127, 481–487 (1990).
Ellis E., Clauser E., Morgan D. O. and Eldery E., Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose.Cell, 45, 721–732 (1986).
Farese R. V., Davis J. S., Barnes D. E., Standaert M. L., Babischkin J. S., Hock R., Rosic N. K., Pollet R. J., The de novo phospholipid effect of insulin is assocated with increases in diacylglycerol, but not inositol phosphates or cytosolic Ca2+.Biochem. J., 231, 269–278 (1985b).
Farese R. V., Standaert M. L., Barnes D. E., Davis J. S. and Pollet R. J., Phorbol ester provokes insuin-like effects on glucose transport, amino acid uptake, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes.Endocrinology, 116, 2650–2655 (1985a).
Freidenberg G. R., Klein H. H., Cordera R., and Olefsky J. M., Insulin receptor kinase activity in rat liver. Regulation by fasting and high carbohydrate feeding.J. Biol. Chem., 260, 12444–12453 (1985).
Gruppuso P. A., Boylan J. M., Levine B. A. and Ellis, L., Insulin receptor tyrosine kinase domain auto-dephosphorylation.Biochem. Biophys. Res. Commun., 189, 1457–1463 (1992).
Heffetz D., Rutter W. J. and Zick Y., The insulinomimetic agent H2O2 and vanadate stimulate tyrosine phosphorylation of potential target proteins for the insulin receptor kinase in intact cells.Biochem. J., 288, 631–635 (1992).
Huang K. P. and Huang F. L., Purification and analysis of protein kinase C isozymes.Method Enzymol., 200, 241–252 (1991).
Jacobs S., Sahyoun N. E., Saltiel A. R. and Cuatrecasas P., Phobol ester stimulate the phosphorylation of receptors for insulin and somatostadin C.Proc. Natl. Acad. Sci. USA, 80, 6211–6213 (1983).
Kadowaki T., Kasuga M., Akanoma Y., Ezaki O. and Takaku F., Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats.J. Biol. Chem., 259, 14208–14216 (1984).
Kasuga M., Kahn C. R., Hedo J. A., E van Obberghen and Yamada K. M., Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation.Proc. Natl. Acad. Sci. USA, 78, 6917–6921 (1981).
Kirsch D., Obermaier B. and Haring H. U., Phorbolesters inhance basal D-glucose transport but inhibit insulin stimulation of D-glucose transport and insulin binding in isolated rat adipocytes.Biochem. Biophys. Res. Commun., 128, 824–832 (1985).
Klein H. H., Freidenberg G. R., Kladde M. and Olefsky J. M., Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. Anin vitro system to measure histone kinase activity of insulin receptors activatedin vivo.J. Biol. Chem., 261, 4691–4697 (1986).
Lee S. H., Moon C. K., Lee M. O. and Kim S. G., Effect of brazifin on glucose oxidation, lipogenes and therein involved enzymes in adipose tissues from diabetic KK mice.Life Science, 53, 1291–1297 (1993).
Moon C. K., Chung J. H., Lee Y. M. and Lee S. H., Effects of brazilin on erythrocyte deformability in streptozotocin induced diabetic rats.Toxicologist, 9, 288–293 (1989).
Moon C. K., Lee S. H., Chung J. H., Won H. S., Kim J. Y., Khil L. Y. and Moon C. H., Effects of brazilin on glucose metabolism in isolated soleus muscles from streptozotocin induced diabetic rats.Arch Pharm, Res., 13, 359–364 (1990).
Moon C. K., Yun Y. P., Lee J. H., Wagner H. and Shin Y. S., Inhibition of lens aldose reductase activity by brazilin and hematoxylin.Planta Medica, 47, 66–67 (1985).
Obermaier B., Ermel B., Klrsch D., Mushack J., Rattenhuber E., Biemer E., Machica Fo and Hearing H. U., Cathechol amines and tumor promoting phorbol esters inhibits insulin receptor kinase and insulin resistance in isolated human adipocytes.Diabetologia, 30, 93–99 (1987).
Petruzzelli L. M., Ganguly S., Smith C. R., Cobb M. H., Rubin C. S. and Rosen O. M., Phosphorylation activates the insulin receptor tyrosine protien kinase.Proc. Natl. Acad. Sci. USA, 80, 3237–3381 (1983).
Pike L. J., Eakes A. T. and Krebs E. G., Characterization of affinity-purified insulin receptor/kinase; Effects of dithiothreitol on receptor/kinase function.J. Biol. Chem., 261, 3782–3789 (1986).
Roach P. J. and Goldman M., Modification of glycogen sythase activity is isolated rat hepatocyte by tumor-promoting phorbol esters: evidence for differental regulation of glycogen synthase and phosphorylase.Proc. Natl. Acad. Sci. USA, 80, 7170–7172 (1983).
Sale G. J., Serine/threonine kinases and tyrosine phosphatases that act on the insulin receptor.Biochem. Soc. Transact., 20, 664–670 (1992).
Smith D. M. and Sale G. J., Characterization of sites of serine phosphorylation in human placental insulin receptor copurified with insulin-stimulated serine kinase activity by two-demensional thin-layer peptide mapping.FEBS Lett., 242, 301–304 (1989).
Smith D. M. and Sale G. J., Evidence that a novel serine kinase catalyses phosphorylation of the insulin receptor in an insulin-dependent and tyrosine kinase-dependent manner.Biochem. J., 256, 903–909 (1988a).
Smith D. M., King M. J. and Sale G. J., Two systemsin vitro that show insulin-stimulated serine kinase activity towards the insulin receptor.Biochem. J., 250, 519–526 (1988b).
Takayama S., White M. F., Lauris V. and Kahn C. R., Phorbol esters modulate insulin receptor phoshorylation and insulin action in cultured hepatoma cells.Proc. Natl. Acad. Sci., USA, 81, 7797–7801 (1984).
Tavare J. M. and Dickens M., Changes in insulin-receptor tyrosine, serine and threonine phosphorylation as a result of substitution of tyrosine-1162 with phenylalnine.Biochem. J., 274, 173–179 (1991).
Tonks N. K., Diltz C. D. and Fischer E. H., Characterization of the major protein-tyrosine-phosphatases of human placenta.J. Biol. Chem., 263, 6731–6738 (1988).
Watarai T., Kobayashi M., Takata Y., Sasaoka T., Iwasaki M. and Shigeta Y., Alteration of insulin receptor kinase activity by high fat feeding.Diabetes, 37, 1397–1404 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, SG., Kim, YM., Khil, LY. et al. Brazilin inhibits activities of protein kinase C and insulin receptor serine kinase in rat liver. Arch. Pharm. Res. 21, 140–146 (1998). https://doi.org/10.1007/BF02974018
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02974018