Abstract
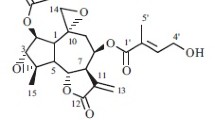
Seven sesquiterpene lactones were isolated by the chromatographic separation of the MeOH extract of the aerial parts ofSaussurea calcicola (Compositae). Their structures were determined spectroscopically to be cynaropicrin (1), arguerin B (2), cebellin F (3), 8α-hydroxy-11α, 13-dihydrozaluzanin C (4), desacylcynaropicrin (5), 3β-hydroxy-8α-epoxymethylacriloiloxy-4(15), 10(14), 11(13)-trien-guaian-6, 12-olide (6), and kandavanolide (7). Compounds1 and2 showed significant cytotoxicity against five cultured human tumor cell lines with ED50 values ranging from 0.23≈1.72 μg/mL.
Similar content being viewed by others
References
Bensky, D. and Gamble, A., “Chinese herbal medicine (Material medica)” Eastland Press, Seattle, pp. 339–340 (1986).
Bohlmann, F. and Gupta, R. K., Further ineupatorolide like germacranolides fromInula cuspidate.Phytochemistry, 21, 157–160 (1982).
Chhabra, B. R., Gupta, S., Jain, M., and Kalsi, P. S., Sesquiterpene lactones fromSaussurea lappa.Phytochemistry, 49, 801–804 (1998).
Cho, J. Y., Baik, K. U., Jung, J. H., and Park, M. H.,In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, fromSaussurea lappa.Eur. J. Pharm., 398, 399–407 (2000).
Dai, J., Zhao, C., Zhang, Q., Liu, Z. L., Zheng, R., and Yang, L., Taraxastane type triterpenoids fromSaussurea petrovii.Phytochemistry, 58, 1107–1111 (2001).
Fernandez, I., Garcia, B., Grancha, F. J., and Pedro, J. R., Two guaianolides fromCentaurea collina.Phytochemistry, 26, 2403–2405 (1987).
Fernandez, I., Garcia, B., Grancha, F. J., and Pedro, J. R., Sesquiterpene lactones, flavonoids and coumarins fromCentaurea collina.Phytochemistry, 28, 2405–2407 (1989).
Gonzalez, A. G., Amaro, J., Fraga, B. M., and Luis, J., 3-Oxo-6β-hydroxyolean-18-enoic acid fromOrthopterygium huancuy.Phytochemistry, 22, 1828–1830 (1983).
Ha, T. J., Jang, D. S., Lee, J. R., Lee, K. D., Hwang, S. W., Jung, H. J., Nam, S. H., Park, K. H., and Yang, M. S., Cytotoxic effects of Sesquiterpene lactones from the flowers ofHemisteptia lyrata B.Arch. Pharm. Res., 26, 925–928 (2003).
Helal, A. M., Nakamura, N., Meselhy, M. R., El-Fishawy, A. M., Hattori, M., and Mahran, G. H., Guaianolides fromCentaurea scoparia.Phytochemistry, 45, 551–554 (1997).
Kisiel, W., 8-Epidesacylcynaropicrin from Crepis capillaris.Planta Med., 49, 246–247 (1983).
Li, Y. and Jia, Z. J., Guaianolides fromSaussurea involucrate.Phytochemistry, 28, 3395–3397 (1989).
Marco, J. A., Sanz, J. F., Albiach, R., Rustaiyan, A., and Habibi, Z., Bisabolene derivatives and sesquiterpene lactones fromcousinia species.Phytochemistry, 32, 395–400 (1993).
Marco, J. A., Sanz-Cervera, J. F., Yuste, A., and Oriola, M. C., Sesquiterpene lactones and dihydroflavonols fromAndryala andUrospermum species.Phytochemistry, 36, 725–729 (1994).
Marco, J. A., Sanz-cervera, J. F., Garcia-lliso, V., Susanna, A., and Garcia-Jacas, N., Sesquiterpene lactones, lignans, and aromatic esters fromCheirolophus species.Phytochemistry, 37, 1101–1107 (1994).
Matsuda, H., Kageura, T., Inoue, Y., Morikawa, T., and Yoshikawa, M., Absolute stereostructures and syntheses of Saussureamines A, B, C, D, and E, amino acid-sesquiterpene conjugates with gastroprotective effect, from the roots ofSaussurea lappa.Tetrahedron, 56, 7763–7777 (2000).
Rustaiyan, A., Niknejad, A., Zdero, C., and Bohlmann, F., A guaianolide fromCentaurea behen.Phytochemistry, 20, 2427–2429 (1981).
Singhal, A. K., Chowdhury, P. K., and Sharma, R. P., Guaianolides fromTricholepis glaberrima.Phytochemistry, 21, 462–463 (1982).
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J. T., Bokesch, H., Kenney, S., and Boyd, M. R., New colorimetric cytotoxicity assay for anticancer-drug screening.J. Natl. Cancer Inst., 82, 1107–1112 (1990).
Youssef, D. and Frahm, A. W.,Circular dichroism of C-7, C-6trans-fused Gualanolides ofCentaurea scoparia.Phtyochemistry, 41, 1107–1111 (1996).
Zdero, C., Bohlmann, F., and Wasshausen, D. C., Guaianolides fromBrachylaena species.Phtyochemistry, 30, 3810–3811 (1991).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S.Z., Choi, S.U. & Lee, K.R. Cytotoxic sesquiterpene lactones fromSaussurea calcicola . Arch Pharm Res 28, 1142–1146 (2005). https://doi.org/10.1007/BF02972976
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02972976