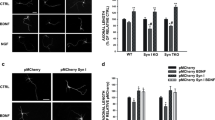
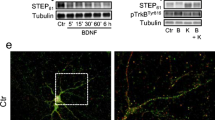
Abstract
The neuronal phosphoprotein B-50/GAP-43 has been implicated in neuritogenesis during developmental stages of the nervous system and in regenerative processes and neuronal plasticity in the adult. The protein appears to be a member of a family of acidic substrates of protein kinase C (PKC) that bind calmodulin at low calcium concentrations. Two of these substrates, B-50 and neurogranin, share the primary sequence coding for the phospho- and calmodulin-binding sites and might exert similar functions in axonal and dendritic processes, respectively. In the adult brain, B-50 is exclusively located at the presynaptic membrane. During neuritogenesis in cell culture, the protein is translocated to the growth cones, i.e., into the filopodia. In view of many positive correlations between B-50 expression and neurite outgrowth and the specific localization of B-50, a role in growth cone function has been proposed. Its phosphorylation state may regulate the local intracellular free calmodulin and calcium concentrations or vice versa. Both views link the B-50 protein to processes of signal transduction and transmitter release.
Similar content being viewed by others
References
Akers R. F. and Routtenberg A. (1985) Protein kinase C phosphorylates a 47 Mr protein (F1) directly related to synaptic plasticity.Brain Res. 334, 147–151.
Albert K. A., Nairn A. C., and Greengard P. (1987) The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization.Proc. Natl. Acad. Sci. USA 84, 7046–7050.
Alexander K. A., Cimler B. M., Meier K. E., and Storm D. R. (1987) Regulation of calmodulin binding to P-57.J. Biol. Chem. 262, 6108–6113.
Alexander K. A., Wakim B. T., Doyle G. S., Walsh K. A., and Storm D. R. (1988) Identification and characterization of the calmodulin-binding domain of neuromodulin, a neurospecific calmodulin-binding protein.J. Biol. Chem. 263, 7544–7549.
Allsopp T. E. and Moss D. J. (1989) A developmentally regulated chicken neuronal protein associated with the cortical cytoskeleton.J. Neurosci. 9, 13–24.
Aloyo V. J., Zwiers H., and Gispen W. H. (1983) Phosphorylation of B-50 protein by calcium-activated, phospholipid-dependent protein kinase and B-50 protein kinase.J. Neurochem. 41, 649–653.
Andreasen T. K., Luetje C. W., Heideman W., and Storm D. R. (1983) Purification of a novel calmodulin binding protein from bovine cerebral cortex membranes.Biochemistry 22, 4615–4618.
Apel E. D., Byford M. F., Douglas A., Walsh K. A., and Storm D. R. (1990) Identification of the protein kinase C phosphorylation site in neuromodulin.Biochemistry 29, 2330–2335.
Apel E. D., Litchfield D. W., Clark R. H., Krebs E. G., and Storm D. R. (1991) Phosphorylation of neuromodulin (GAP-43) by casein kinase II: identification of phosphorylation sites and regulation by calmodulin.J. Biol. Chem. 266, 10,544–10,551.
Au D. C., Apel E. D., Chapman E. R., Estep R. P., Nicolson T. A., and Storm D. R. (1989) Expression of cDNAs encoding wild-type and mutant neuromodulins inEscherichia coli: comparison with the native protein from bovine brain.Biochemistry 28, 8142–8148.
Baetge E. E. and Hammang J. P. (1991) Neurite outgrowth in PC12 cells deficient in GAP-43.Neuron 6, 21–30.
Baizer L., Alkan S., Stocker K., and Ciment G. (1990) Chicken growth-associated protein (GAP)-43: primary structure and regulated expression of mRNA during embryogenesis.Mol. Brain Res. 7, 61–68.
Banno Y., Nagao S., Katada T., Nagata K., Ui M., and Nozawa Y. (1987) Stimulation by GTP-binding proteins (Gi, Go) of partially purified phospholipase C activity from human platelet membranes.Biochem. Biophys. Res. Comm. 146, 861–869.
Basi G. S., Jacobson R. D., Virág I., Schilling J., and Skene J. H. P. (1987) Primary structure and transcriptional regulation of GAP-43, a protein associated with nerve growth.Cell 49, 785–791.
Baudier J., Bronner C., Kligman D., and Cole R. D. (1989) Protein kinase C substrates from bovine brain. Purification and characterization of neuromodulin a neuron-specific calmodulin-binding protein.J. Biol. Chem. 264, 1824–1828.
Baudier J., Deloulme J. C., Van Dorsselaer A., Black D., and Matthes H. W. D. (1991) Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17).J. Biol. Chem. 266, 229–237.
Benowitz L. I. and Lewis E. R. (1983) Increased transport of 44,000 to 49,000-dalton acidic proteins during regeneration of the goldfish optic nerve.J. Neurosci. 3, 2153–2163.
Benowitz L. I., Perrone-Bizzozero N. I., and Finklestein S. P. (1987) Molecular properties of the growth-associated protein GAP-43 (B-50).J. Neurochem. 48, 1640–1647.
Benowitz L. I. and Routtenberg A. (1987) A membrane phosphoprotein associated with neural development, axonal regeneration, phospholipid metabolism, and synaptic plasticity.Trends Neurosci. 10, 527–532.
Benowitz L. I., Apostolides P. J., Perrone-Bizzozero N. I., Finklestein S. P., and Zwiers H. (1988) Anatomical distribution of the growth associated proteins GAP-43/B-50 in the adult rat brain.J. Neurosci. 8, 339–352.
Benowitz L. I., Perrone-Bizzozero N. I., Finklestein S. P., and Bird E. D. (1989) Localization of the growth-associated phosphoprotein GAP-43 (B-50, F1) in the human cerebral cortex.J. Neurosci. 9, 990–995.
Benowitz L. I., Rodriguez W. R., and Neve R. I. (1990) The pattern of GAP-43 immunostaining changes in the rat hippocampal formation during reactive synaptogenesis.Mol. Brain Res. 8, 7–23.
Biffo S., Verhaagen J., Schrama L. H., Schotman P., Danho W., and Margolis F. L. (1990) B-50/GAP43 expression correlates with process outgrowth in the embryonic mouse nervous system.Eur. J. Neurosci. 2, 487–499.
Brown A. M. and Birnbaumer L. (1990) Ionic channels and their regulation by G protein subunits.Ann. Rev. Physiol. 52, 197–213.
Burry R. W., Lah J. J., and Hayes D. M., (1991) Redistribution of GAP-43 during growth cone development in vitro: immunocytochemical studies.J. Neurocytol. 20, 133–144.
Chan S. Y., Murakami K., and Routtenberg A. (1986) Phosphoprotein F1: purification and characterization of a brain kinase C substrate related to plasticity.J. Neurosci. 6, 3618–3627.
Changelian P. S., Meiri K. F., Soppet D., Valenza H., Loewy A., and Willard M. B. (1990) Purification of the growth-associated protein GAP-43 by reversed phase chromatography: amino acid sequence analysis and cDNA identification.Brain Res. 510, 259–268.
Chapman E. R., Au D., Alexander A., Nicolson T. A., and Storm D. R. (1991) Characterization of the calmodulin binding domain of neuromodulin.J. Biol. Chem. 266, 207–213.
Cimler B. M., Andreasen T. J., Andreasen K. I., and Storm D. R. (1985) P-57 is a neural specific calmodulin-binding protein.J. Biol. Chem. 260, 10,784–10,788.
Cimler B. M., Giebelhaus D. H., Wakim B. T., Storm D. R., and Moon R. T. (1987) Characterization of murine cDNAs encoding P-57, a neural-specific calmodulin-binding protein.J. Biol. Chem. 262, 12,158–12,163.
Coggins P. J. and Zwiers H. (1989) Evidence for a single protein kinase C-mediated phosphorylation site in rat brain protein B-50.J. Neurochem. 53, 1895–1901.
Coggins P. J., McIntyre D. D., Vogel A. J., and Zwiers H. (1989) Neuronal protein B-50: a proton nuclear-magnetic-resonance study.Biochem. Soc. Trans. 17, 785–786.
Coggins P. J. and Zwiers H. (1990) Binding of the neuronal protein B-50, but not the metabolite B-60, to calmodulin.J. Neurochem. 54, 274–277.
Coggins P. J. and Zwiers H. (1991) B-50 (GAP-43): biochemistry and functional neurochemistry of a neuron-specific phosphoprotein.J. Neurochem. 56, 1095–1106.
Coggins P. J., Stanisz J., Nagy A., and Zwiers H. (1991) Identification of a calmodulin-binding, B-50-immunoreactive C-kinase substrate (BICKS) in bovine brain.Neurosci. Res. Commun. 8, 49–56.
Colbran R. J. and Soderling T. R. (1990) Calcium/calmodulin dependent protein kinase II.Curr. Topics Cell Reg. 31, 181–217.
Costello B., Lin L-H., Meymandi A., Bock S., Norden J. J., and Freeman J. A. (1991) Expression of the growth and plasticity-associated neuronal protein, GAP-43, in PC12 pheochromocytoma cells.Prog. Brain Res. 89, 47–67.
Da Cunha A. and Vitkovic L. (1990) Regulation of immunoreactive GAP-43 expression in rat cortical macroglia is cell type specific.J. Cell. Biol. 111, 209–215.
De Graan P. N. E., Van Hooff C. O. M., Tilly B. C., Oestreicher A. B., Schotman P., and Gispen W. H. (1985) Phosphoprotein B-50 in nerve growth cones from fetal rat brain.Neurosci. Lett. 61, 235–241.
De Graan P. N. E., Dekker L. V., De Witt M., Schrama L. H., and Gispen W. H. (1988a) Modulation of B-50 phosphorylation and phosphoinositide metabolism in synaptic plasma membranes by protein kinase C, phorbol esters and ACTH.J. Recep. Res. 8, 345–361.
De Graan P. N. E., Heemskerk F. M. J., Dekker L. V., Melchers B. P. C., Gianotti C., and Schrama L. H. (1988b) Phorbol esters induce long and short-term enhancement of B-50/GAP-43) phosphorylation in rat hippocampal slices.Neurosci. Res. Commun. 3, 175–182.
De Graan P. N. E., Oestreicher A. B., De Wit M., Kroef M., Schrama L. H., and Gispen W. H. (1990) Evidence for the binding of calmodulin to endogenous B-50 (GAP-43) in native synaptosomal plasma membranes.J. Neurochem. 55, 2139–2141.
De Graan P. N. E., Oestreicher A. B., Schotman P., and Schrama L. H. (1991) Protein kinase C substrate B-50 (GAP-43) and neurotransmitter release.Prog. Brain Res. 89, 187–207.
Dekker L. V., De Graan P. N. E., Oestreicher A. B., Versteeg D. H. G., and Gispen W. H., (1989a) Inhibition of noradrenaline release by antibodies to B-50 (GAP-43).Nature 342, 74–76.
Dekker L. V., De Graan P. N. E., Versteeg D. H. G., Oestreicher A. B., and Gispen W. H. (1989b) Phosphorylation of B-50 (GAP43) is correlated with neurotransmitter release in rathippocampal slices.J. Neurochem. 52, 24–30.
Dekker L. V., De Graan P. N. E., De Wit., Hens J. J. H., and Gispen W. H. (1990a) Depolarization-induced phosphorylation of the protein kinase C substrate B-50 (GAP-43) in rat cortical synaptosomes.J. Neurochem. 54, 1645–1652.
Dekker L. V., De Graan P. N. E., Spierenburg H., De Wit M., Versteeg D. H. G., and Gispen W. H. (1990b) Evidence for a relationship between B-50 (GAP-43) and [3H]noradrenaline release in rat brain synaptosomes.Eur. J. Pharmacol. 188, 113–122.
Dekker L. V., De Graan P. N. E., and Gispen W. H. (1991) Transmitter release: target of regulation by protein kinase C?Prog. Brain Res. 89, 209–233.
De la Monte S., Federoff H. J., Ng S.-C., Grabczyk E., and Fishman M. C. (1989) GAP-43 gene expression during development: persistence in a distinctive set of neurons in the mature central nervous system.Dev. Brain Res. 46, 161–168.
Deloulme J. C., Janet T., Au D., Storm D. R., Sensenbrenner M., and Baudier J. (1990) Neuromodulin (GAP43): a neuronal protein kinase C substrate is also present in 0–2A glial cell lineage. Characterization of neuro-modulin in secondary cultures of oligodendrocytes and comparison with the neuronal antigen.J. Cell Biol. 111, 1559–1569.
DiFiglia M., Roberts R. C., and Benowitz L. L. (1990) Immunoreactive GAP43 in the neuropil of adult rat neostriatum: localization in unmyelinated fibres, axon terminals, and dendritic spines.J. Comp. Neurol. 302, 992–1001.
Dokas L. A., Pisano M. R., Schrama L. H., Zwiers H., and Gispen W. (1990) Dephosphorylation of B-50 in synaptic plasma membranes.Brain Res. Bull. 24, 321–329.
Dolphin A. C., Errington M. L., and Bliss T. V. P. (1982) Long-term potentiation of the perforant pathin vivo is associated with increased glutamate release.Nature 297, 496–498.
Dosemeci A. and Rodnight R. (1987) Demonstration by phase-partitioning in Triton X-114 solutions that phosphoprotein B-50 (F-1) from rat brain is an integral membrane protein.Neurosci. Lett. 74, 325–330.
Dotti C. G., Banker G. A., and Binder L. I. (1987) The expression and distribution of the microtubule-associated proteins tau and microtubule-associated protein 2 in hippocampal neurons in the ratin situ and in cell culture.Neuroscience 23, 121–130.
Dotti C. G., Sullivan C. A., and Banker G. A. (1988) The establishment of polarity by hippocampal neurons in culture.J. Neurosci. 8, 1454–1468.
Erzurumlu R. S., Jhaveri S., Moya K. L., and Benowitz L. I. (1989) Peripheral nerve regeneration induces elevated expression of GAP-43 in the brainstem trigeminal complex of adult hamsters.Brain. Res. 498, 135–139.
Erzurumlu R. S., Jhaveri S., and Benowitz L. I. (1990) Transient patterns of GAP-43 expression during the formation of barrels in the rat somatosensory cortex.J. Comp. Neurol. 292, 443–456.
Estep R. P., Alexander K. A., and Storm D. R. (1990) Regulation of free calmodulin levels in neurons by neuromodulin: relationship to neuronal growth and regeneration.Curr. Topics Cell Reg. 31, 161–180.
Gianotti C., Nunzi M. G., Gispen W. H., and Corradetti R. (1991) B-50 phosphorylation following different patterns of electrical stimulation in rat hippocampal slices.Synaptic Plasticity Meeting, Mainz, Germany.
Gilbert W. (1986) Genes-in-pieces revisited.Science 228, 823–824.
Gilman A. G. (1987) G proteins: transducers of receptor-generated signals.Ann. Rev. Biochem. 56, 615–649.
Gispen W. H. (1986) Phosphoprotein B-50 and phosphoinoitide in brain synaptic plasma membranes: a possible feedback relationship.Biochem. Soc. Trans. 14, 163–165.
Gispen W. H., Leunissen J. L. M., Oestreicher A. B., Verkleij A. J., and Zwiers H. (1985a) Presynaptic localization of B-50 phosphoprotein: the ACTH-sensitive protein kinase C substrate involved in rat brain phosphoinositide metabolism.Brain Res. 328, 381–385.
Gispen W. H., Van Dongen C. J., De Graan P. N. E., Oestreicher A. B., and Zwiers H. (1985b) The role of phosphoprotein B-50 in phosphoinositide metabolism in brain synaptic plasma membranes, inInositol and Phosphoinositides, Bleasdale J. E., Hauser G., and Eichberg J., eds., Humana, Clifton, NJ, pp. 399–413.
Gispen W. H., De Graan P. N. E., Chan S. Y., and Routtenberg A. (1986) Comparison between the neural acidic proteins B-50 and F1.Prog. Brain Res. 69, 383–386.
Gordon-Weeks P. R. (1989) GAP-43—What does it do in the growth cone.Trends Neurosci. 12, 363–365.
Gorgels T. G. M. F., Van Lookeren Campagne M., Oestreicher A. B., Gribnau A. A. M., and Gispen W. H. (1989) B-50/GAP-43 is localized at the cytoplasmic side of the plasma membrane in developing and adult rat pyramidal tract.J. Neurosci. 9, 3861–3869.
Goslin K., Schreyer D. J., Skene J. H. P., and Banker G. (1988) Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones.Nature 366, 672–680.
Goslin K. and Banker G. (1990) Rapid changes in the distribution of GAP-43 correlate with the expression of neuronal polarity during normal development and under experimental conditions.J. Cell Biol. 110, 1319–1331.
Goslin K., Schreyer D. J., Skene J. H. P., and Banker G. (1990) Changes in the distribution of GAP-43 during development of neuronal polarity.J. Neurosci. 10, 588–602.
Grabzcyk E., Zuber M. X., Federoff H. J., Ng S.-C., Pack A., and Fishman M. C. (1990) Cloning and characterization of the rat gene encoding GAP-43.Eur. J. Neurosci. 2, 822–827.
Graff J. M., Stumpo D. J., and Blackshear P. J. (1989a) Characterization of the phosphorylation sites in the chicken and bovine myristoylated alanine-rich C kinase substrate protein, a prominent cellular substrate for protein kinaseC. J. Biol. Chem. 264, 11,912–11,919.
Graff J. M., Young T. N., Johnson J. D., and Blackshear P. J. (1989b) Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinaseC. J. Biol. Chem. 264, 21,818–21,823.
Greene L. A. and Shooter E. M. (1980) the nerve growth factor: biochemistry, synthesis and mechanism of action.Ann. Rev. Neurosci. 3, 353–402.
Hammang J. P., Messing A., and Baetge E. E. (1990) The C6 glioma cell line expresses the growth-associated protein GAP-43 in a developmentally regulated fashion.Soc. Neurosci. Abstr. 16, 339.8.
Heemskerk F. M. J., Schrama L. H., and Gispen W. H. (1989) Activation of protein kinase C by 4-aminopyridine dependent on Na+ channel activity in rat hippocampal slices.Neurosci. Lett. 106, 315–321.
Heemskerk F. M. J., Schrama L. H., De Graan P. N. E., and Gispen W. H. (1990) 4-Aminopyridine stimulates B-50 (GAP-43) phosphorylation in rat synaptosomes.J. Mol. Neurosci. 2, 11–17.
Hoffman P. N. (1989) Expression of GAP-43, a rapidly transported growth-associated protein, and class II β-tubulin, a slowly transported cytoskeletal protein, are coordinated in regenerating neurons.J. Neurosci. 9, 893–897.
Howell T. W., Cockcroft S., and Gomperts B. D. (1987) Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized mast cells.J. Cell Biol. 105, 191–197.
Houbre D., Duportail G., Deloulme J-C., and Baudier J. (1991) The interactions of the brain-specific calmodulin-binding protein kinase C substrate, neuromodulin (GAP-43), with membrane phospholipids.J. Biol. Chem. 266, 7121–7131.
Karms L. R., Ng S.-C., Freeman J. A., and Fishman M. C. (1987) Cloning of complementary DNA for GAP-43, a neuronal growth-related protein.Science 236, 597–600.
Katz F., Ellis L., and Pfenninger K. H. (1985) Nerve growth cones isolated from fetal rat brain III: calcium-dependent protein phosphorylation.J. Neurosci. 5, 1402–1411.
Kleinschmidt J. A., Dingwall C., Maier G., and Franke W. W. (1986) Molecular characterization of a karyophilic, histone-binding protein: cDNA cloning, amino acid sequence and expression of nuclear protein N1/N2 ofXenopus laevis.EMBO J. 5, 3547–3552.
Kosik K. S., Orecchio L. D., Bruns G. A. P., Benowitz L. I., MacDonald G. P., Cox D. R., and Neve R. L. (1988) Human GAP-43: its deduced amino acid sequence and chromosomal localization in mouse and human.Neuron 1, 127–132.
Kozak M. (1989) The scanning model for translation: an update.J. Cell Biol. 108, 229–241.
Labate M. E. and Skene J. H. P. (1989) Selective conservation of GAP-43 structure in vertebrate evolution.Neuron 3, 299–310.
Levine J., Skene J. H. P., and Willard M. B. (1981) GAPs and fodrin. Novel axonally transported proteins.Trends Neurosci. 4, 273–277.
Linden D. J. and Routtenberg A. (1989) The role of protein kinase C in long-term potentiation: a testable model.Brain Res. Rev. 14, 279–296.
Liu Y. and Storm D. R. (1989) Dephosphorylation of neuromodulin by calcineurin.J. Biol. Chem. 264, 12,800–12,804.
Liu Y. and Storm D. R. (1990) Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration.Trends Pharmacol. Sci. 11, 107–111.
Lovinger D. M., Colley P. A., Akers R. F., Nelson R. B., and Routtenberg A. (1986) Direct relation of long-term synaptic potentiation to phosphorylation of membrane protein F1, a substrate for membrane protein kinase C.Brain Res. 399, 205–211.
Low M. G. (1987) Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors.Biochem. J. 22, 1–13.
Magee T. and Hanley M. (1988) Sticky fingers and CAAX boxes.Nature 335, 114–115.
Masure H. R., Alexander K. A., Wakim B. T., and Storm D. R. (1986) Physicochemical and hydrodynamic characterization of P-57, a neurospecific calmodulin binding protein.Biochemistry 25, 7553–7560.
McDonald J. R. and Lawrence J. C. (1989) Identification of an adipocyte protein that binds to calmodulin in the absence of Ca2+ and is phosphorylated in response to insulin and tumor-promoting phorbol esters.J. Biol. Chem. 262, 9611–9618.
McIntosh H., Daw N., and Parkinson D. (1990) GAP-43 in the cat visual cortex during postnatal development.Visual. Neurosci. 4, 585–593.
McMaster D., Zwiers H., and Lederis K. (1988) The growth associated neuronal phosphoprotein B-50: improved purification, partial primary structure and characterization and localization of proteolysis products.Brain Res. Bull. 21, 265–276.
Megerian J. T. and Klein W. L. (1990) Evidence that neuromodulin (GAP-43, B-50, pp46, F1) is not essential for early neuritogenesis.Soc. Neurosci. Abstr. 16, 339.11.
Meiri K. F., Pfenninger K. H., and Willard M. B. (1986) Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones.Proc. Natl. Acad. Sci. USA 83, 3537–3541.
Meiri K. F., Willard M. B., and Johnson M. I. (1988) Distribution and phosphorylation of the growthassociated protein GAP-43 in regenerating sympathetic neurons in culture.J. Neurosci. 8, 2571–2581.
Meiri K. F. and Gordon-Weeks P. R. (1990) GAP-43 in growth cones is associated with areas of membrane that are tightly bound to substrate and is a component of a membrane skeleton subcellular fraction.J. Neurosci. 10, 256–266.
Meiri K. F., Bickerstaff L. E., and Schwob J. E. (1991) Monoclonal antibodies show that kinase C phosphorylation of GAP-43 during axonogenesis is both spatially and temporally restricted in vivo.J. Cell. Biol. 112, 991–1005.
Miller R. J. (1986) Protein kinase C: a key regulator of neuronal excitability?Trends Neurosci. 9, 538–541.
Moss D. J., Fernyhough P., Chapman K., Baizer L., Bray D., and Allsopp T. (1990) Chicken growthassociated protein GAP-43 is tightly bound to the actin-rich neuronal membrane skeleton.J. Neurochem. 54, 729–736.
Moya K. L., Benowitz L. I., and Schneider G. E. (1990) Abnormal retinal projections alter GAP-43 patterns in the diencephalon.Brain Res. 527, 259–265.
Nelson R. B., Linden D. J., Hyman C., Pfenninger K. H., and Routtenberg A. (1989) The two major phosphoproteins in growth cones are probably identical to two protein kinase C substrates correlated with persistence of long-term potentiation.J. Neurosci. 9, 381–389.
Neve R. L., Perrone-Bizzozero N. I., Finklestein S., Zwiers H., Bird E., Kurnit D. M., and Benowitz L. I. (1987) The neuronal growth-associated protein GAP-43 (B-50, F1): neuronal specificity, developmental and regulation and regional distribution of the human and rat mRNAs.Mol. Brain Res. 2, 177–183.
Neve R. L., Finch E. A., Bird E. D., and Benowitz L. I. (1988) Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain.Proc. Natl. Acad. Sci. USA 85, 3638–3642.
Neve R. L., Dawes L. R., and Geller A. I. (1990) Fusion of the amino-terminal 10 amino acids of GAP-43 to β-galactosidase targets the chimeric protein to neuronal processes.Soc. Neurosci. Abstr. 16, 27.6.
Ng S.-C., De la Monte S. M., Conboy G. L., Karns L. R., and Fishman M. C. (1988) Cloning of human GAP-43: growth association and ischemic resurgence.Neuron 1, 133–139.
Nielander H. B., Schrama L. H., Van Rozen A. J., Kasperaitis M., Oestreicher A. B., De Graan P. N. E., Gispen W. H., and Schotman P. (1987) Primary structure of the neuronspecific phosphoprotein B-50 is idendcal to growth-associated protein GAP-43.Neurosci. Res. Commun. 1, 163–172.
Nielander H. B., Schrama L. H., Van Rozen A. J., Oestreicher A. B., Gispen W. H., and Schotman P. (1989) The calmodulin-binding protein B-50/GAP-43: localization of the proteolytic sites forSlaureus protease and the phosphorylation sites for protein kinase C.J. Neurochem. S52, S106A.
Nielander H. B., Schrama L. H., Van Rozen A. J., Kasperaitis M., Oestreicher A. B., Gispen W. H., and Schotman P. (1990) Mutation of serine 41 in the neuron-specific protein B-50 (GAP-43) prohibits phosphorylation by protein kinase C.J. Neurochem. 55, 1442–1445.
Nishizuka Y. (1988) The molecular heterogeneity of protein kinase C and its implications for cellular regulation.Nature 334, 661–665.
Nishizuka Y. (1989) The family of protein kinase C for signal transduction.JAMA 262, 1826–1833.
Oestreicher A. B., Van Dongen C. J., Zwiers H., and Gispen W. H. (1983) Affinity-purified anti-B-50 protein antibody: interference with the function of the phosphoprotein B-50 in synaptic plasma membranes.J. Neurochem. 41, 331–340.
Oestreicher A. B. and Gispen W. H. (1986) Comparison of the immunocytochemical distribution of the phosphoprotein B-50 in the cerebellum and hippocampus of immature and adult rat brain.Brain Res. 375, 267–279.
Oestreicher A. B., Dekker L. V., and Gispen W. H. (1986) A radioimmunoassay for the phosphoprotein B-50: distribution in rat brain.J. Neurochem. 46, 1366–1369.
Oestreicher A. B., De Graan P. N., Schrama L. H., Lamme V. A. F., Bloemen R. J., Schotman P., and Gispen W. H. (1989) The protein kinase C phosphosite(s) in B-50 (GAP-43) are confined to 15K phosphofragments produced byStaphylococcus aureus V8 protease.Neurochem. Int. 14, 361–372.
O'Hara B. F., Fisher S., Oster-Granite M. L., Gearhart J. D., and Reeves R. H. (1989) Developmental expression of the amyloid precursor protein, growth-associated protein 43, and somatostatin in normal and trisomy 16 mice.Dev. Brain Res. 49, 300–304.
O'Neil K. T. and Degrado W. F. (1990) How calmodulin binds its targets: sequence independent recognition of amphiphilic α-helices.Trends Biochem. Sci. 15, 59–64.
Perrone-Bizzozero N. I., Weiner D., Hauser G., and Benowitz L. I. (1988) Extraction of major acidic Ca2+ dependent phosphoproteins from synaptic membranes.J. Neurosci. Res. 20, 346–350.
Perrone-Bizzozero N. I., Benowitz L. I., Apostolides P. I., Franck E. R., Finklestein S. P., and Bizzozero O. A. (1989) Protein fatty acid acylation in developing cortical neurons.J. Neurochem. 52, 1149–1155.
Pfenninger K. H. (1986) Of nerve growth cones, leukocytes and memory: second messenger systems and growth-regulated proteins.Trends Neurosci. 9, 562–565.
Pisano M. R., Hegazy M. G., Reimann E. W., and Dokas L. A. (1988) Phosphorylation of protein B-50 (GAP-43) from adult rat brain cortex by casein kinase II.Biochem. Biophys. Res. Commun. 155, 1207–1212.
Ramakers G. J. A., Oestreicher A. B., Van Leeuwen F. W., De Graan P. N. E., and Gispen W. H. (1991) Developmental changes in B-50 (GAP-43) in primary cultures of cerebral cortex: B-50 immunolocalization, axonal elongation rate and growth cone morphology.Int. J. Dev. Neurosci. 9, 231–241.
represa A., Deloulme J. C., Sensenbrenner M., Ben-Ari Y., and Baudier J. (1990) Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate.J. Neurosci. 10, 3782–3792.
Rodnight R. (1982) Aspects of protein phosphorylation in the nervous system with particular reference to synaptic transmission.Prog. Brain Res. 56, 1–25.
Rosenthal A., Chan S. Y., Henzel W., Haskell C., Kuang W.-J., Chen E., Wilcox J. N., Ullrich A., Goeddel D. V., and Routtenberg A. (1987) Primary structure and mRNA localization of protein F1, a growth-related protein kinase C substrate associated with synaptic plasticity.EMBO J. 6, 3641–3646.
Schmidt M. F. G. (1983) Fatty acid binding: a new kind of posttranslational modification of membrane proteins.Curr. Topics Microbiol. Immunol. 102, 101–112.
Schotman P., Nielander H. B., Van Rozen A. J., Frankena H., Schrama L. H., and Gispen W. H. (1989) Microheterogeneity of the growth-associated neuronal protein B-50 (GAP-43). Contribution of phosphorylation by protein kinase C.J. Chromatogr. 483, 301–309.
Schotman P., Jap Tjoen San E. R. A., Schmidt-Michels M., and Gispen W. H. (1990) Antisense B-50 (GAP-43) prevents neurite outgrowth in PC12 cells.Soc. Neurosci. Abstr. 16, 339.5.
Schrama L. H., De Graan P. N. E., Oestreicher A. B., and Gispen W. H. (1987) B-50 phosphorylation, protein kinase C and the induction of excessive grooming behavior in the rat.Adv. Exp. Med. Biol. 221, 393–409.
Schrama L. H., Heemskerk F. M. J., and De Graan P. N. E. (1989) Dephosphorylation of protein kinase C phosphorylated B-50/GAP-43 by the calmodulin-dependent phosphatase calcineurin.Neurosci. Res. Commun. 5, 141–147.
Schrama L. H., Nielander H. B., Van Rozen A. J., Schotman P., and Gispen W. H. (1990) Organization of the rat and the human B-50 (GAP-43) gene.Soc. Neurosci. Abstr. 16, 154.11.
Sheu F. S., Kasamatsu T., and Routtenberg A. (1990) Protein kinase-C activity and substrate (F1/GAP-43) phosphorylation in developing cat visual cortex.Brain Res. 24, 144–148.
Skene J. H. P. and Willard M. B. (1981) Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems.J. Cell Biol. 89, 96–103.
Skene J. H. P. (1989) Axonal growth-associated proteins.Ann. Rev. Neurosci. 12, 127–156.
Skene J. H. P. and Virág I. (1989) Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43.J. Cell Biol. 108, 613–624.
Stein R., Mori N., Matthews K., Lo L.-C., and Anderson D. J. (1988) The NGF-inducible SCG10 mRNA encodes a novel membrane-bound protein present in growth cones and abundant in developing neurons.Neuron 1, 463–476.
Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., and Fishman M. C. (1990) Go is a major growth cone protein subject to regulation by GAP-43.Nature 344, 836–841.
Takano E., Maki M., Mori H., Hatanaka M., Marti T., Titani K., Kannagi R., Ooi T., and Murachi T. (1988) Pig heart calpastatin: identification of repetitive domain structures and anomalous behavior in polyacrylamide gel electrophoresis.Biochemistry 27, 1964–1972.
Tetzlaff W., Zwiers H., Lederis K., Cassar L., and Bisby M. A. (1989) Axonal transport and localization of B-50/GAP-43-like immunoreactivity in regenerating sciatic and facial nerves of the rat.J. Neurosci. 9, 1303–1313.
Van der Neut R., Oestreicher A. B., Gispen W. H., and Bär P. R. (1990) The expression of B-50/GAP-43 during development of rat spinal neurons in culture is regulated by interneuronal contact.Neurosci. Lett. 109, 36–41.
Van der Zee C. E. E. M., Nielander H. B., Vos J. P., Lopes da Silva S., Verhaagen J., Oestreicher A. B., Schrama L. H., Schotman P., and Gispen W. H. (1989) Expression of growth-associated protein B-50 (GAP43) in dorsal root ganglia and sciatic nerve during regenerative sprouting.J. Neurosci. 9, 3505–3512.
Van Dongen C. J., Zwiers H., Oestreicher A. B., and Gispen W. H. (1985) ACTH, phosphoprotein B-50, and polyphosphoinositide metabolism in rat brain membranes, inPhospholipids in the Nervous System, vol. 2. Horrocks L. A., ed., Raven, New York, pp. 49–59.
Van Hooff C. O. M., De Graan P. N. E., Oestreicher A. B., and Gispen W. H. (1988) B-50 phosphorylation and polyphosphoinositide metabolism in nerve growth cone membranes.J. Neurosci. 8, 1789–1795.
Van Hooff C. O. M., Oestreicher A. B., De Graan P. N. E., and Gispen W. H. (1989a) Role of the growth cone in neuronal differentiation.Mol. Neurobiol. 3, 101–133.
Van Hooff C. O. M., Holthuis J., Oestreicher A. B., Boonstra J., De Graan P. N. E., and Gispen W. H. (1989b) Nerve growth factor-induced changes in the intracellular localization of the protein kinase C substrate B-50 in pheochromocytoma cells.J. Cell Biol. 108, 1115–1125.
Van Lookeren Campagne M., Oestreicher A. B., Van Bergen en Henegouwen P. M. P., and Gispen W. H. (1990) Ultrastructural immunocytochemical localization of B-50/GAP-43, a protein kinase C substrate, in isolated presynaptic nerve terminals and neuronal growth cones.J. Neurocytol. 18, 479–489.
Van Lookeren Campagne M., Oestreicher A. B. Buma P., Verkleij A. J., and Gispen W. H., (1991a) Ultrastructural localization of adrenocorticotrophic hormone and the phosphoprotein B-50/growth-associated protein 43 in freeze-substituted, lowicryl HM20-embedded mesencephalic central gray substance of the rat.Neuroscience 42, 517–529.
Van Lookeren Campagne M., Dotti C. G., Jap Tjoen San E. R. A., Gispen W. H., and Oestreicher A. B. (1991b) Ultrastructural distribution of B-50/GAP-43 in cultured hippocampal neurons during various developmental stages.Soc. Neurosci. Abstr. 17, 525.17.
Verhaagen J., Oestreicher A. B., Gispen W. H., and Margolis F. L. (1989) The expression of the growth associated protein B-50/GAP43 in the olfactory system of neonatal and adult rats.J. Neurosci. 9, 683–691.
Verhaagen J., Oestreicher A. B., Grillo M., Khew-Goodall, Y.-S., Gispen W. H., and Margolis F. L. (1990) Neuroplasticity in the olfactory system: differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of B-50/GAP43 and the olfactory marker protein.J. Neurosci. Res. 26, 31–44.
Vitkovic L., Steisslinger H. W., Aloyo V. J., and Mersel M. (1988) The 43-kDa neuronal growth-associated protein (GAP-43) is present in plasma membranes of rat astrocytes.Proc. Natl. Acad. Sci. USA 85, 8296–8300.
Wakim B. T., Alexander K. A., Masure H. R., Cimler B. M., Storm D. R., and Walsh K. A. (1987) Amino acid sequence of P-57, a neurospecific calmodulinbinding protein.Biochemistry 26, 7466–7470.
Watson J. B., Battenberg E. F., Wong K. K., Bloom F. E., and Sutcliffe J. G. (1990), Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein.J. Neurosci. Res. 26, 397–408.
Widmer F. and Caroni P. (1990) Identification, localization, and primary structure of CAP-23, a particle-bound cytosolic protein of early development.J. Cell Biol. 11, 3035–3047.
Woolf C. J., Reynolds M. L., Molander C., O'Brien C., Lindsay R. M., and Benowitz L. I. (1990) The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following peripheral nerve injury.Neuroscience 34, 465–478.
Yamamoto M. and Kondo H. (1990) Gene expression of a neuronal growth-associated protein, GAP-43, in the paraganglionic carotid body as well as in the autonomic ganglia of normal adult rats.Neurosci. Lett. 117, 275–279.
Yankner B. A., Benowitz L. I., Villa-Komaroff L., and Neve R. L. (1990) Transfection of PC12 cells with the human GAP-43 gene: effects on neurite out-growth and regeneration.Mol. Brain Res. 7, 39–44.
Zuber M. X., Strittmatter S. M., and Fishman M. C. (1989a) A membrane-targeting signal in the amino terminus of the neuronal protein GAP-43.Nature 341, 345–348.
Zuber M. X., Goodman D. W., Karns L. R., and Fishman M. C. (1989b) The neuronal growth-associated protein GAP-43 induces filopodia in nonneuronal cells.Science 224, 1193–1195.
Zwiers H., Veldhuis H. D., Schotman P., and Gispen W. H. (1976) ACTH, cyclic nucleotides, and brain protein phosphorylationin vitro.Neurochem. Res. 1, 669–677.
Zwiers H., Wiegant V. M., Schotman P., and Gispen W. H. (1978) ACTH-induced inhibition of endogenous rat brain protein phosphorylationin vitro: structure activity.Neurochem. Res. 3, 455–463.
Zwiers H., Tonnaer J., Wiegant V. M., Schotman P., and Gispen W. H. (1979) ACTH-sensitive protein kinase from rat brain membranes.J. Neurochem. 33, 247–256.
Zwiers H., Schotman P., and Gispen W. H. (1980) Purification and some characteristics of an ACTH-sensitive protein kinase and its substrate protein in rat brain membranes.J. Neurochem. 34, 1689–1699.
Zwiers H., Gispen W. H., Kleine L., and Mahler H. R. (1982) Specific proteolysis of a brain membrane phosphoprotein (B-50): effects of calcium and calmodulin.Neurochem. Res. 7, 127–137.
Zwiers H., Verhaagen J., Van Dongen C. J., De Graan P. N. E., and Gispen W. H. (1985) Resolution of rat brain synaptic phosphoprotein B-50 into multiple forms by twodimensional electrophoresis: evidence for multisite phosphorylation.J. Neurochem. 44, 1083–1090.
Zwiers H. and Coggins P. J. (1990) Corticotropin (ACTH) inhibits the specific proteolysis of the neuronal phosphoprotein B-50/GAP-43.Peptides 11, 951–954.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gispen, W.H., Nielander, H.B., De Graan, P.N.E. et al. Role of the growth-associated protein B-50/GAP-43 in neuronal plasticity. Mol Neurobiol 5, 61–85 (1991). https://doi.org/10.1007/BF02935540
Issue Date:
DOI: https://doi.org/10.1007/BF02935540