Abstract
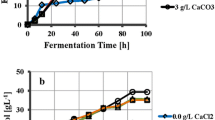
The addition of a limited concentration of yeast extract to a minimal salt medium (MSM) enhanced cell growth and increased the production of curdlan whereas nitrogenlimitation was found to be essential for the higher production of curdlan byAgrobacterium sp. ATCC 31749. As the amount of the inoculum increased, the cell growth as well as the production of curdlan also increased in the MSM without a nitrogen source. The cell growth and production of curdlan increased as the initial pH of the medium decreased as low as 5.0. The conversion rate and concentration of curdlan from 2% (w/v) glucose in the MSM with concentrated cells under nitrogen deletion was 67% and 13.4 g/L, respectively. The highest conversion rate of curdlan under the conditions optimized in this study was 71% when the glucose concentration was 1% (w/v).
Similar content being viewed by others
References
Harada, T., K. Fujimori, S. Hirose, and M. Masada (1966) Crowth and β-1,3 glucan 10C3K production by a mutant ofAlcaligenes faecalis var.myxogenes in defined medium.Agr. Biol. Chem. 30: 764–769.
Harada, T., A. Misaki, and H. Saito (1968) Curdlan: A bacterialgel-forming β-1,3-glucan.Arch. Biochem. 124: 292–298.
Maeda, I., H. Saito, M. Masada, A. Misaki, and T. Harada (1967) Properties of gels formed by heat treatment of curdlan, a bacterial β-1,3 glucan.Agr. Biol. Chem. 31: 1184–1188.
Harada, T., M. Masada, K. Fujimori, and I. Maeda (1966) Production of a firm, resilient gel-forming polysaccharide by a mutant ofAlcaligenes faecalis var.myxogenes 10C3.Agr. Biol. Chem. 30: 196–198.
Ayers, S. H. and P. Rupp (1920) Extracts of pure dry yeast for culture media.J. Bacteriol. 5: 89–98.
Harada, T., M. Masada, K. Fujimori, and I. Maeda (1966) Production of firm, resilient gel-forming polysaccharide in natural medium by a mutant ofAlcaligenes faecalis var.myxogenes 10C3.J. Ferment. Technol. 44: 20–24.
Phillips, K. R. and H. C. Lawford (1983) Curdlan: its properties and production in batch and continuous fermentation.Prog. Ind. Microbiol. 18: 201–229.
Lawford, H. G., K. R. Phillips, and G. R. Lawford (1982) A two stage continuous process for the production of thermogelable curdlan-type expolysaccharide.Biotechnol. Lett. 4: 689–694.
Lee, I. Y., W. T. Seo, G. J. Kim, C. S. Park, and Y. H. Park (1997) Production of curdlan of using sucrose or sugar cane molasses by two-step fed-batch cultivation ofAgrobacterium species.J. Ind. Microbiol. Biotechnol. 18: 255–259.
Seviour, R. J. and B. Kristiansen (1983) Effect of ammonium ion concentration on polysaccharide production byAureobasidium pullulans in batch culture.Eur. J. Appl. Microbiol. Biotechnol. 17: 178–181.
Ko, S. H., H. S. Lee, S. H. Park, and H. K. Lee (2000) Optimal conditions for the production of exopolysaccharide by marine microorganismHahella chejuensis.Biotechnol. Bioprocess Eng. 5: 181–185.
Orts, W. J., J. D. Rousseau, and H. G. Lawfor ((1987) Improved microbial production of curdlan type-polysaccharide. pp. 459–469. In: S. Stivala, V. Crescenzi, and I. C. M. Dea (eds.).Industrial Polysaccharides. Gordon Breach Science, New York, USA.
Phillips, K. R., J. Pik, H. G. Lawford, B. Lavers, A. Kligerman, and G. R. Lawford (1983) Production of curdlantype polysaccharide byAlcaligenes faecalis in batch and continuous culture.Can. J. Microbiol. 29: 1331–1338.
Lawford, H. G. (1982) Continuous process for the production of gelable exopolysaccharide.US Patent 4,355,106.
Lawford, H. G. and J. D. Rousseau (1992) Production of β-1,3-glucan exopolysaccharide in low shear systems.Appl. Biochem. Biotechnol. 34/35: 597–612.
Lee, J. W., W. G. Yeomans, A. F. Allen, D. L. Kaplan, F. Deng, and R. A. Gross (1997) Exopolymers from curdlan production: incorporation of glucose-related sugars byAgrobacterium sp. strain ATCC 31749.Can. J. Microbiol. 43: 149–156.
Lee, J. W., W. G. Yeomans, A. F. Allen, D. L. Kaplan, and R. A. Gross (1997) Microbial production of water-soluble non curdlan type exopolymer-B with controlled composition byAgrobacterium sp.Biotechnol. Lett. 19: 1217–1221.
Chaplin, M. (1982) A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography.Anal. Biochem. 123: 336–341.
Sharmila, M., K. Ramanans, and N. Sethunathan (1989) Effect of yeast extract on the degradation of organophosphorous insecticides by soil enrichment and bacterial cultures.Can. J. Microbiol. 35: 1105–1110.
Shen, C. F., N. Kosaric, and R. Blaszezyk (1993) Properties of anaerobic sludge as affected by yeast extract, cobalt and iron suppleents.Appl. Microbiol. Biotechnol. 39: 132–137.
Kim, M. K., I. Y. Lee, J. H. Ko, Y. H. Rhee, and Y. H. Park (1999) Higher intracellular levels of uridinemonophosphate under nitrogen-limited conditions enhance metabolic flux of curdlan synthesis inAgrobacterium species.Biotechnol. Bioeng. 62: 317–323.
Ebbole, D. J. (1998) Carbon catabolite repression of gene expression and condition inNeurospora crassa.Fungal Gen. Biol. 25: 15–21.
Anwar, M. N., M. Suto, and F. Tomita (1996) Isolation of mutants ofPenicillium purpurogen resistant to catabolite repression.Appl. Microbiol. Biotechnol. 45: 684–687.
Wolff, J. A., C. H. MacGregor, R. C. Eisenberg, and P. V. Phibbs Jr. (1991) Isolation and characterization of catabolite repression control mutants ofPseudomonas acruginosa PAO.J. Bacteriol. 173: 4700–4706.
Gancedo, J. M. (1998) Yeast carbon catabolite repression.Microbiol. Mol. Biol. Rev. 62: 334–361.
Lee, I. Y., M. K. Kim, W. T. Lee, J. K. Seo, H. W. Jung, and Y. H. Park (1999) Influence of agitation speed on production of curdlan byAgrobacterium species.Bioproess Eng. 20: 283–287.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, DY., Cho, YS., Chung, CH. et al. Improved production of curdlan with concentrated cells ofAgrobacterium sp.. Biotechnol. Bioprocess Eng. 6, 107–111 (2001). https://doi.org/10.1007/BF02931955
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931955