Abstract
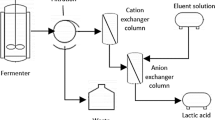
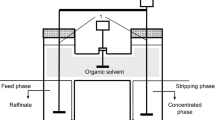
A nondispersive extraction process for recovery of lactic acid from fermentation broth is being developed. The criteria for selection of solvent, distribution of lactic acid between the aqueous and solvent phases, and the effect of presence of other compounds in the broth, are discussed. Working with a simulated fermentation broth (without cells), a hydrophobic membrane module has been evaluated for its effectiveness as extractor. Back extraction and its role has been demonstrated. A theoretical comparison of this process with electrodialysis shows membrane extraction to be more desirable.
Similar content being viewed by others
Abbreviations
- ad :
-
Specific surface area of alkali droplets, cm2/cm3
- Ao :
-
Mass transfer surface area in membrane module, m2
- Ac :
-
Total cross-sectional area of fiber tubes, cm2
- C1 :
-
Concentration of lactic acid in the inlet solvent stream to extractor, g/L
- C2 :
-
Concentration of lactic acid in the outlet solvent stream from extractor, g/L
- do :
-
Outside diameter of membrane fibers, cm
- dp :
-
Average diameter of membrane fibers, cm
- FB :
-
Volumetric flow rate of broth, L/h
- Fs :
-
Volumetric flow rate of solvent, L/h
- kc :
-
Mass transfer coefficient between solvent and alkali phase in the solvent tank, cm/s
- kc :
-
Mass transfer coefficient between solvent and alkali phase in the fiber tubes, cm/s
- ko :
-
Over-all mass transfer coefficient across the membrane in the extractor, cm/s
- m:
-
Distribution coefficient, dimensionless
- m' :
-
Distribution coefficient of lactic acid at the pH of alkali solution, dimensionless
- m* :
-
Partition coefficient of lactic acid, dimensionless
- P1 :
-
Concentration of lactic acid in the inlet aqueous stream to extractor, g/L
- P2 :
-
Concentration of lactic acid in the outlet aqueous stream from extractor, g/L
- Pa :
-
Concentration of lactic acid in alkali phase, g/L
- VI :
-
Volume of aqueous phase, L
- VII :
-
Volume of solvent (TOPO+kerosene) phase, L
- VIII :
-
Volume of alkali phase emulsified in the solvent, L
- ØD :
-
Volume fraction of alkali in the solvent emulsion, L
References
Lipinsky, E. S. and Sinclair, R. G. (1986),Chem. Eng. Prog. 82(8), 26.
Viniegra-Gonzalez, G. and Gomez, J. (1984),Bioconversion Systems, Wise, D. L. ed., CRC, Boca Raton, FL, pp. 17–39.
Holten, C. H., Mueller A., and Rehbinder, G. (1971),Lactic Acid—Properties and Chemistry of Lactic Acid and Derivatives, Verlag Chemie, Weinheim, Ger.
Harrero, A. A. (1983),Trends in Biotechnol. 1, 49.
Kertes, A. S. and King, C. J. (1986),Biotechnol. Bioeng. 28, 269.
Yabannavar, V. M. and Wang, D. I. C. (1987),Ann. NY Acad. Sci.. 506, 523.
Hongo, M., Nomura, Y., and Iwahara, M. (1986),Appl. Environ. Microbiol. 52, 314.
Nomura, Y., Iwahara, M., and Hongo, M. (1988),Biotechnol. Bioeng. 30, 788.
Boyaval, P., Corre, C, and Terre, S. (1987),Biotechnol. Lett. 9, 207.
Tsao, G. T. (1988), Paper presented at the 2nd Corn Utilization Cofn., Oct. 18–19, Columbus, OH.
Golob, J., Grilic, V., and Zadnik, B. (1981),I & EC Proc. Des. Dev. 20, 433.
Ricker, N. L., Pittman, E. F., and King, C. J. (1980),J. Separ. Proc. Technol. 1, 23.
Cheng, P. S. (1989), MS Thesis, Chemical Eng. Dept, University of Missouri-Columbia, MO.
Playne, M. J. and Smith, B. R. (1983),Biotechnol. Bioeng. 25, 1251.
Hayward, G. and Lau, I. (1989),Can. J. Chem. Eng. 67, 157.
Dutta, R. (1981),Biotechnol. Bioeng. 23, 61.
Roffler, S. R., Blanch, H. W., and Wilke, C. R. (1984),Trends in Biotechnol. 2, 129.
Bar, R. and Gainer, J. L. (1987),Biotechnol. Progr. 3, 109.
Bajpai, R. K., Iannotti, E. L., Wang, C. J., and Su, B. (1990), AIChE national meeting, paper no. 62e, Orlando, FL, March 18–22.
Saravanan, G. and Srinivasan, D. (1985),J. Chem. Eng. Data 30, 166.
Eisen, E. O. and Joffee, J. (1966),J. Chem. Eng. Data 11, 480.
Shah, D. J. and Tiwari, K. K. (1981),J. Chem. Eng. Data 26, 375.
Prasad, R. and Sirkar, K. K. (1988),AIChE J. 34, 177.
Prasad, R. and Sirkar, K. K. (1987),AIChE J. 33, 1057.
Skelland, A. H. P. (1974),Diffusional Mass Transfer, John Wiley, NY.
Calderbank, P. H. (1967),Mixing: Theory and Practice, Uhl, V. W. and Grey, J. B. (eds.), vol. 2, Academic, NY.
Yeh, P. and Bajpai, R. K. (1989), 19th Annual Biochemical Engineering Symposium, University of Missouri-Columbia, MO, April 22.
Luedeking, R. and Piret, E. L. (1959),J. Biochem. Microbiol. Tech. Eng. 1, 393.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, C.J., Bajpai, R.K. & Iannotti, E.L. Nondispersive extraction for recovering lactic acid. Appl Biochem Biotechnol 28, 589–603 (1991). https://doi.org/10.1007/BF02922635
Issue Date:
DOI: https://doi.org/10.1007/BF02922635