Abstract
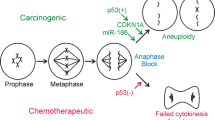
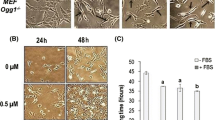
Arsenic is a well-established carcinogen in humans, but there is little evidence for its carcinogenicity in animals and it is inactive as an initiator or tumor promoter in two-stage models of carcinogenicity in mice. Studies with cells in culture have provided some possible mechanisms by which arsenic and arsenical compounds may exert a carcinogenic activity. Sodium arsenite and sodium arsenate were observed to induce morphological transformation of Syrian hamster embryo cells in a dose-dependent manner. The trivalent sodium arsenite was greater than tenfold more potent than the pentavalent sodium arsenate. The compounds also exhibited toxicity; however, transformation was observed at nontoxic as well as toxic doses. At low doses, enhanced colony forming efficiency of the cells was observed. To understand the mechanism of arsenic-induced transformation, the genetic effects of the two arsenicals were examined over the same doses that induced transformation. No arsenic-induced gene mutations were detected at two genetic loci. However, cell transformation and cytogenetic effects, including endoreduplication, chromosome aberrations, and sister chromatid exchanges, were induced by the arsenicals with similar dose responses. These results support a possible role for chromosomal changes in arsenic-induced transformation. The two arsenic salts also induced another form of mutation-gene amplification. Both sodium arsenite and sodium arsenate induced a high frequency of methotrexate-resistant 3T6 cells, which were shown to have amplified copies of the dihydrofolate reductase gene. The ability of arsenic to induce gene amplification may relate to its carcinogenic effects in humans since amplification of oncogenes is observed in many human tumors. Epidemiological studies suggest that arsenic acts late in the carcinogenic process in humans and oncogene amplification correlates with the progression of tumors. These observations lead us to propose the hypothesis that arsenic acts as a tumor progressor, rather than a tumor initiator or tumor promoter. Arsenic-induced chromosome aberrations or gene amplifications may play a role in tumor progression.
Similar content being viewed by others
References
IARC Monograph on Evaluation of Carcinogenic Risk to Humans 23, 1980, p. 37.
IARC Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to Humans Suppl. 4, 1982, p. 50.
A. Leonard and R. R. Lauwerys,Mutat. Res. 75, 49 (1980).
N. Ishinishi, A. Yamamoto, A. Hisanga, and T. Inamasu,Cancer Lett. 21, 141 (1983).
P. Rudnay and M. Borzsonyi,Magyar Onkologia 25, 73 (1981).
G. Pershagen, G. Nordberg, and N. Bjorklund,Environ. Res. 34, 227 (1984).
J. A. DiPaolo and B. C. Casto,Cancer Res. 30, 1008 (1979).
T. C. Lee, M. Oshimura, and J. C. Barrett,Carcinogenesis 6, 1421, (1985). figures © AAAS.
T. C. Lee, N. Tanaka, P. W. Lamb, T. M. Gilmer, and J. C. Barrett,Science 241, 79–81 (1988), figures © AAAS.
Zhang, Q. and J. C. Barrett,Toxic. in Vitro. Toxic. in Vitro. 2: 303 (1988).
S. A. Lerman, T. W. Clarkson, and R. J. Gerson,Chem. Biol. Interactions 45, 401 (1983).
M. Vahter and E. Marafante,Chem. Biol. Interactions 47, 29 (1983).
T. G. Rossman, D. Stone, M. Molina, and W. Troll,Environ. Mut. 2, 371 (1980).
L. L. Deaven and A. E. Nock,J. Cell. Biol. 83, 159a (1979).
J. Petres, D. Baron, and M. Hagedon,Environ. Hlth. Perspect. 19, 223 (1977).
M. L. Larramendy, N. C. Popescu, and J. A. DiPaolo,Environ. Mut. 3, 597 (1981).
K. Nakamuro and Y. Sayato,Mutat. Res. 88, 73 (1981).
G. R. Paton and A. C. Allison,Mutat. Res. 16, 332 (1972).
B. Wan, R. T. Christian, and S. W. Soukup,Environ. Mut. 4, 493 (1982).
T. D. Tlsty, P. C. Brown and R. T. Schimke,Mol. Cell Biol. 4, 1050 (1984).
P. C. Brown, T. D. Tlsty, and R. T. Schimke,Mol. Cell Biol. 3, 1097 (1983).
R. T. Schimke,Cell 37, 705 (1984).
A. B. Hill and R. T. Schimke,Cancer Res. 45, 5050 (1985).
A. Varshavsky,Proc. Natl. Acad. Sci. 78, 3673 (1981).
R. K. Boutwell,J. Agric. Food Chem. 11, 381 (1963).
C. Baroni, G. J. van Esch, and V. Saffiotti,Arch. Environ. Health 7, 668 (1963).
C. C. Brown and K. Chu,J. Natl. Cancer Inst. 70, 455 (1983).
D. L. George,Cancer Surveys 3, 497 (1984).
M. Schwab et al.,Proc. Natl. Acad. Sci. 81, 4940 (1984).
G. M. Brodeur, R. C. Seeger, M. Schwab, H. E. Varmus, and J. M. Bishop,Science 224, 1121 (1984).
J. C. Barrett,Carcinogenesis, vol. 8, M. J. Mass et al., eds., Raven, NY, 1985, p. 423.
H. Hennings et al.,Nature 304, 67 (1983).
J. F. O’Connell, A. J. P. Klein-Szanto, D. M. DiGiovanni, J. W. Fries, and T. J. Slaga,Cancer Res. 46, 2863 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barrett, J.C., Lamb, P.W., Wang, T.C. et al. Mechanisms of arsenic-induced cell transformation. Biol Trace Elem Res 21, 421–429 (1989). https://doi.org/10.1007/BF02917284
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02917284