Abstract
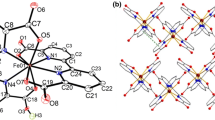
The structure of two trinuclear iron acetates [Fe3O(CH3COO)6(H2O)3]Cl· 6H2O (I) and [Fe3O(CH3COO)6(H2O)3][FeCl4] · 2CH3COOH (II) was determined by X-ray diffraction analysis. Crystals I and II are ionic and belong to the orthorhombic system with parameters a = 13.704(3), b = 23.332(5), c = 9.167(2) Å, R = 0.0355, space goup P21212 for I and a = 10.145(4), b = 15.323(6), c = 22.999(8) Å, R = 0.0752, space group Pbc21 for II. The complex cation [Fe3O(CH3COO)6(H2O)3]+ has a μ3-O-bridged structure typical for trinuclear iron (III) compounds. As shown by Mössbauer spectroscopy, the iron(III) ions are in the high-spin state. In trinuclear cations, antiferromagnetic exchange interaction takes place between the Fe(III) ions with the exchange parameter J = -26.69 cm−1 for II (Heisenberg-Dirac-Van Vleck model for D3h, symmetry).
Similar content being viewed by others
References
M. A. Porai-Koshits,Itogi Nauiki Tekh., Ser. Kristallokhim.,15, 3–129 (1981).
B. O. West,Polyhedron,8, No. 3, 219–274 (1989).
E. L. Muettertis,Catal. Rev.,23, Nos. 10-2, 69–81 (1981).
R. C. Mehrota and R. Bohra,Metal Carboxylates, Academic Press, London (1983).
R. D. Cannon and R. P. White,Prog. Inorg. Chem.,36, 196–297 (1988).
K. K. Crichton,Inorganic Biochemistry of Iron Metabolism, New York (1991).
S. I. Lippard,Angew. Chem. Int. Ed. Engl,27, 344–361 (1988).
K. Wieghardt, K. Pohl, I. Jibril, and C. Huttner,Angew. Chem.,96, 66–67 (1984).
K. L. Taft, G. C. Papaefthymiou, and S. J. Lippard,Science,259, 1302–1305 (1993).
W. Micklitz and S. J. Lippard,J. Am. Chem. Soc.,111, No. 17, 6856–6858 (1989).
C. I. Turta, S. T. Shova, F. A. Spatar’, et al.,Zh. Strukt. Khim.,35, No. 2, 112–120 (1994).
S. G. Shova, I. G. Cadelnic, F. C. Jovmir, et al.,Koordinats. Khim.,23, No. 9, 672–678 (1997).
C. I. Turta, A. G. Lazéresku, Yu. A. Simonov, et al.,ibid,22, No. 1, 45–53 (1996).
I. G. Cadelnic, S. G. Shova, Yu. A. Simonov, et al.,Polish J. Chem.,71, 501–508 (1997).
F. A. Spatar’, V. M. Meriacre, V. E. Zubareva, et al.,Koordinats. Khim.,22, No. 3, 188–193 (1996).
V. T. Kalinnikov and Yu. V. Rakitin,Introduction to Magnetochemistry [in Russian], Mir, Moscow (1991).
G. M. Sheldrick,Acta Crystallogr.,46A, 467–473 (1990).
G. M. Sheldrick,SHELXL-93. Program for the Refinement of Crystal Structure, University of Göttingen, Germany (1993).
K. Anzenhofer and J. J. De Boer,Rec. Trav. Chim. Pays-Bas,88, No. 3, 286–288 (1969).
R. V. Pound and G. A. Rebka,Phys. Rev. Lett.,4, Nos. 5–6, 274–275 (1960).
N. N. Greenwood and T. C. Gibb,Mössbauer Spectroscopy, Charman and Hall, London (1971).
A. Earnshaw, B. N. Figgis, and J. Lewis,J. Chem. Soc. (A), 1656–1663 (1966).
G. J. Long, W. T. Robinson, W. P. Tappmeyer, and D. L. Bridges,J. Chem. Soc., Dalton Trans., No. 6, 573–579 (1973).
M. Takano,J. Phys. Soc. Jpn.,33, No. 5, 1312–1317 (1972).
B. D. Rumbold and G. V. H. Wilson,J. Phys. Chem. Solids,34, No. 11, 1887–1891 (1973).
Author information
Authors and Affiliations
Additional information
Translated fromZhumal Struktumoi Khimii, Vol. 39, No. 5, pp. 917–933, September–October, 1998.
Rights and permissions
About this article
Cite this article
Shova, S.G., Cadelnic, I.G., Gdaniec, M. et al. Syntheses and structural study of trinuclear iron acetates [Fe3O(CH3COO)6(H2O)3]Cl· 6H2O and [Fe3O(CH3COO)6(H2O)3] [FeCl4]· 2CH3COOH. J Struct Chem 39, 747–761 (1998). https://doi.org/10.1007/BF02903548
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02903548